XB-IMG-127834
Xenbase Image ID: 127834
|
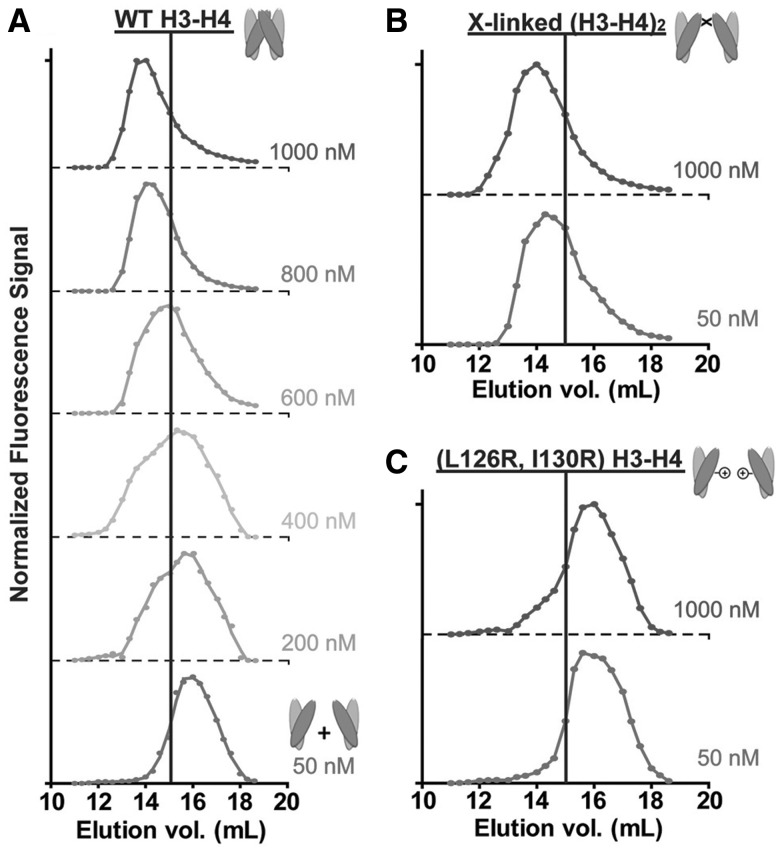
Figure 3. The oligomeric state of the histone H3-H4 complex is dynamic across the nanomolar range. The size-exclusion elution profile for wild-type H3-H4 gradually shifts toward the right upon dilution from 1000 nM to 50 nM. At 1000 nM, the H3-H4 complex elutes at a volume (14 ml) consistent with a tetrameric conformation. At 50 nM, the H3-H4 peak elutes at 16 ml, consistent with a dimeric state. Elution profiles for cross-linked (H3-H4)2 tetramers remain at 14 ml at both 1000 and 50 nM (top right panel). The H3(L126R, I130R)-H4 double mutation prohibits H3:H3′ four-helix bundle formation and thus precludes (H3-H4)2 tetramer formation. This mutant histone complex elutes at 16 ml for both high and low concentrations (bottom right panel). Image published in: Winkler DD et al. (2012) © The Author(s) 2012. This image is reproduced with permission of the journal and the copyright holder. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial license Larger Image Printer Friendly View |
