XB-IMG-136412
Xenbase Image ID: 136412
|
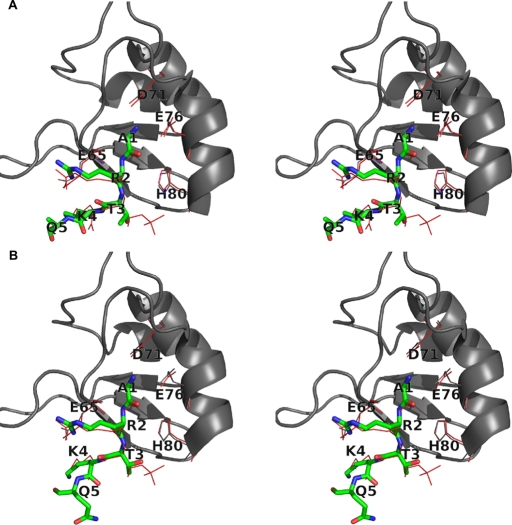
FIGURE 2:. Survivin interacts with the unmodified H3 tail in similar way as with H3T3ph. (A) Superposition of the crystal structure of wild-type Survivin with H3T3ph(1-4) (red lines) on the structure of wild-type Survivin (gray; cartoon representation) with H3(1-12) peptide (green in stick representation). For clarity, only D71, D76, H80, E65, and both peptides are shown in line or stick representation. Figures are in stereo representation. (B) Superposition of the crystal structure of wild-type Survivin with H3T3ph(1-4) (red lines) on the structure of K62A Survivin (gray; cartoon representation) with H3(1-12) peptide (green in stick representation). For clarity only, D71, D76, H80, E65, and both peptides are shown in line or stick representation. T3 Oγ can form a weak hydrogen bond with the H80 side chain if present in the modeled orientation. Alternatively, the hydrophobic interactions between T3 Cγ and Survivin may be formed if T3 adopts a different orientation (not shown). Determination of the prevailing orientation of T3 was not possible due to the low quality of its corresponding electron density. Figures are in stereo representation. Image published in: Niedzialkowska E et al. (2012) © 2012 Niedzialkowska et al. This image is reproduced with permission of the journal and the copyright holder. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
