XB-IMG-172084
Xenbase Image ID: 172084
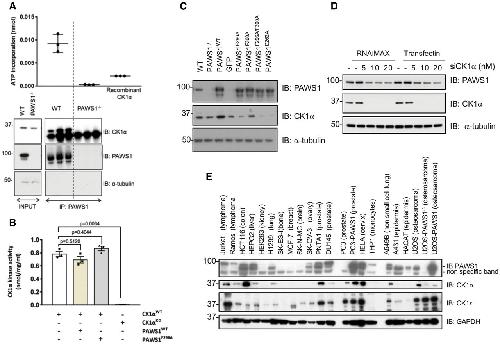
|
|
Figure 6.
PAWS1 does not affect CK1α activity but affects the protein levels of CK1α in cells
Endogenous PAWS1 was immunoprecipitated from wild‐type and PAWS1−/− U2OS cells (n = 3), and the associated CK1α kinase activity was measured following a kinase assay using γ32P‐ATP and the CK1tide peptide substrate. A third of the PAWS1 IP samples were resolved by SDS–PAGE and immunoblotted with the indicated antibodies (bottom panel) (n = 3, error bars represent ± SEM).
In vitro wild‐type (WT) or catalytically inactive (KD) CK1α kinase assay using CK1tide as a substrate in the presence or absence of recombinant PAWS1WT or PAWS1F296A (n = 3; error bars represent ± SEM; one way ANOVA with multiple comparisons, n.s.: no statistical significance).
Cell extracts from U2OS wild‐type, PAWS1−/− and PAWS1−/− cells in which PAWS1, GFP control, PAWS1F296A, PAWS1F300A, PAWS1F296A/F300A or PAWS1D262A was restored, were resolved by SDS–PAGE and immunoblotted with the indicated antibodies.
CK1α expression was silenced in U2OS cells with indicated amounts of siRNA using two different transfection reagents (RNAi‐Max or TransFectin) and PAWS1 protein levels monitored by Western blotting.
Expression of PAWS1 and CK1α protein in the indicated cell lines was monitored by Western blotting. The protein levels of CK1α mirror the levels of PAWS1 in the majority of the cancer cell lines tested. Image published in: Bozatzi P et al. (2018) © 2018 The Authors. This image is reproduced with permission of the journal and the copyright holder. This is an open-access article distributed under the terms of the Creative Commons Attribution license Larger Image Printer Friendly View |
