CRISPR/Cas9 mediated knockout of rb1 and rbl1 leads to rapid and penetrant retinoblastoma development in Xenopus tropicalis
Naert et al. Science Reports (2016)
Abstract
Retinoblastoma is a pediatric eye tumor in which bi-allelic inactivation of the Retinoblastoma 1 (RB1) gene is the initiating genetic lesion. Although recently curative rates of retinoblastoma have increased, there are at this time no molecular targeted therapies available. This is, in part, due to the lack of highly penetrant and rapid retinoblastoma animal models that facilitate rapid identification of targets that allow therapeutic intervention. Different mouse models are available, all based on genetic deactivation of both Rb1 and Retinoblastoma-like 1 (Rbl1), and each showing different kinetics of retinoblastoma development. Here, we show by CRISPR/Cas9 techniques that similar to the mouse, neither rb1 nor rbl1 single mosaic mutant Xenopus tropicalis develop tumors, whereas rb1/rbl1 double mosaic mutant tadpoles rapidly develop retinoblastoma. Moreover, occasionally presence of pinealoblastoma (trilateral retinoblastoma) was detected. We thus present the first CRISPR/Cas9 mediated cancer model in Xenopus tropicalis and the first genuine genetic non-mammalian retinoblastoma model. The rapid kinetics of our model paves the way for use as a pre-clinical model. Additionally, this retinoblastoma model provides unique possibilities for fast elucidation of novel drug targets by triple multiplex CRISPR/ Cas9 gRNA injections (rb1 + rbl1 + modifier gene) in order to address the clinically unmet need of targeted retinoblastoma therapy.
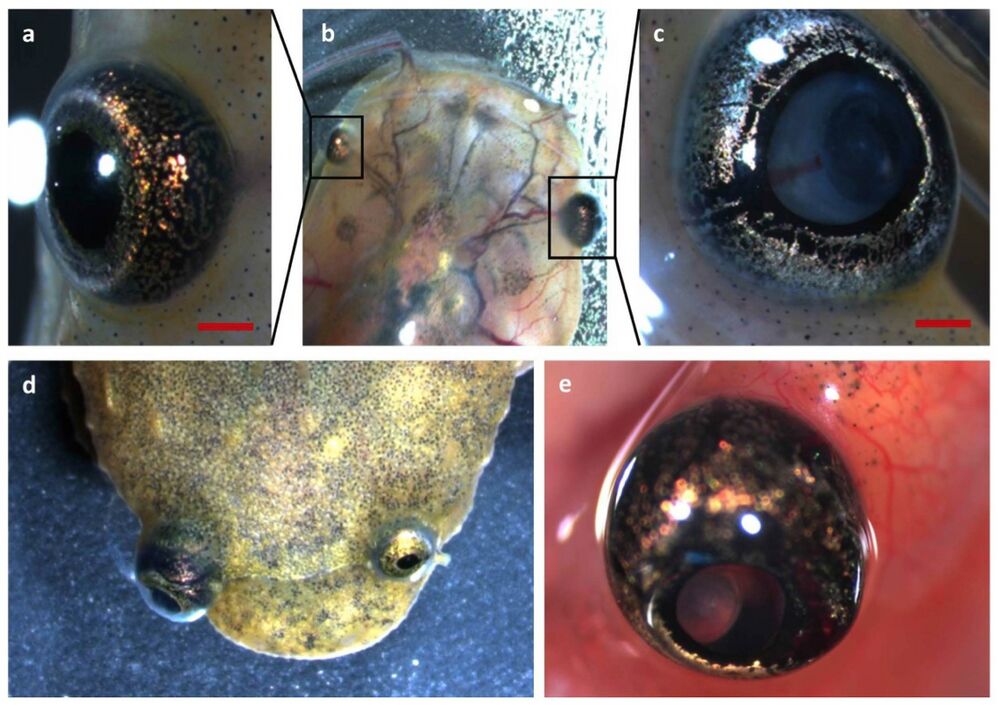
Figure 1. Retinoblastoma 1 and retinoblastoma-like 1 CRISPR/Cas9 injected Xenopus tropicalis develop retinoblastoma. (a–c) Development of unilateral retinoblastoma can be externally observed in tadpoles injected with rb1 and rbl1 gRNAs, when comparing the eye on the uninjected side with the eye on the injected side in this tadpole. Leukocoria and ectopia lentis are apparent. (d) Unilateral retinoblastoma in a froglet. (e) Externally visible tumor-associated neovasculature.
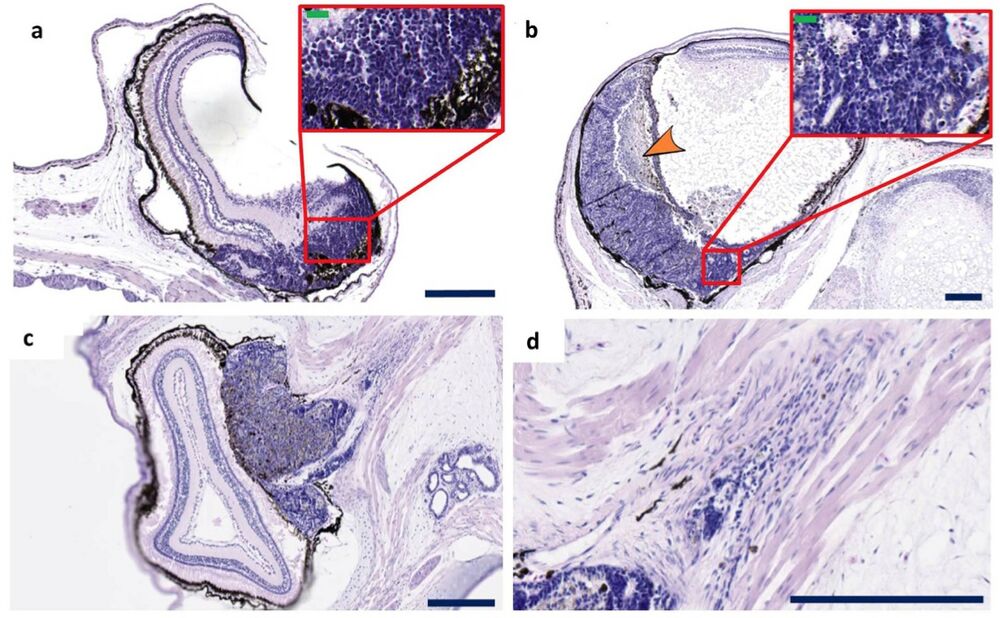
Figure 2. Histopathology of retinoblastomas. (a–d) Examples of retinoblastomas observed in tadpoles and froglets injected with rb1 and rbl1 gRNAs. Under low magnification, retinoblastoma appears as a large basophilic mass with pink and purple foci. These tumor masses arise from and destroy the retina and can either fill part of the vitreous cavity, thus following an endophytic growth pattern (a,b) including necrotic areas (orange arrowhead), or can grow in the outer layers of the retina (exophytic growth pattern) (c). The poorly differentiated neuroblastic cells appear blue because they have intensely basophilic nuclei and scanty cytoplasm (‘small blue round cell tumor’) (inset a,b). The poorly differentiated neuroblastic cells show varying degrees of retinal differentiation with formation of (Homer Wright) rosettes. The tumors with exophytic growth can invade in the optic nerve (d). Scale bar sizes as follows; Blue scale bars are 200 μm. Green scale bars are 20 μm. Sections have been cut longitudinal.
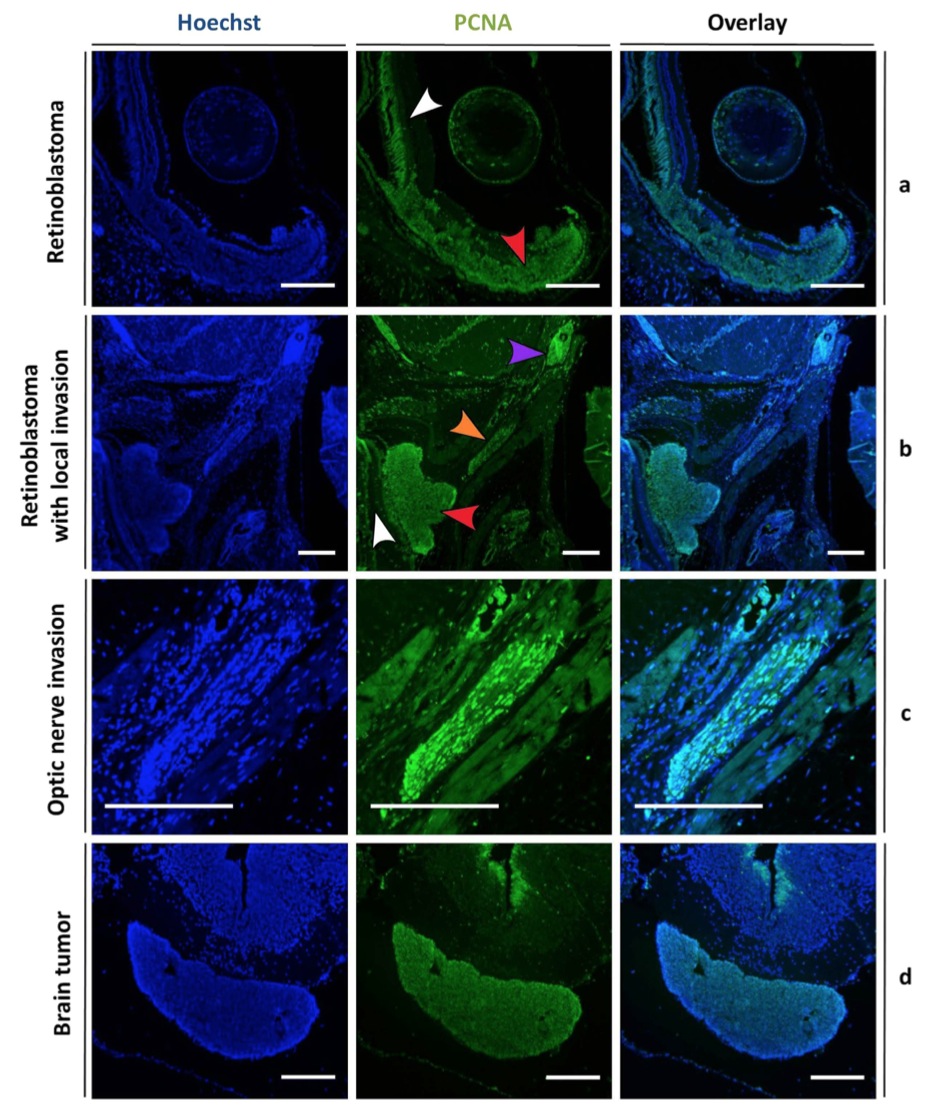
Figure 5. Staining for a proliferation marker demonstrates aggressive growth characteristics of tumor cells in rb1cr1/rbl1cr1 MDKO tadpoles. Hoechst (left panels) and proliferating cell nuclear antigen (PCNA) (middle panels) double staining, with overlay in right panel. (a,b) PCNA staining is detected within the retinoblastoma (red arrowhead) whilst the adjacent normal retina remains quiescent (white arrowhead). In (b), tumor cells invading into the optic nerve (orange arrowhead) and a more distant metastasis (purple arrow) can be distinguished. (c) Higher magnification of the optic nerve clearly reveals PCNA positive tumor cells. (d) PCNA staining is uniformly detected within the brain tumor, whereas the surrounding normal brain tissue remains quiescent, with the exception of the subventricular zone. Scale bars are 200 μM.
Click here to view this article at Science Reports.
Click here to view this article on Xenbase.
Click here to view this article on Pubmed.
Adapted with permission from Macmillan Publishers Ltd: Naert et al. (2016).CRISPR/Cas9 mediated knockout of rb1 and rbl1 leads to rapid and penetrant retinoblastoma development in Xenopus tropicalis. Sci Rep. 2016 Oct 14;6:35264. doi: 10.1038/srep35264, copyright (2016).
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
