Mechanosensing is critical for axon growth in the developing brain
Koser et al. Nature Neuroscience (2016) doi:10.1038/nn.4394
Click here to view this article at Nature Neuroscience.
Click here to view this article on Xenbase.
Click here to view this article on Pubmed.
Abstract
During nervous system development, neurons extend axons along well-defined pathways. The current understanding of axon pathfinding is based mainly on chemical signaling. However, growing neurons interact not only chemically but also mechanically with their environment. Here we identify mechanical signals as important regulators of axon pathfinding. In vitro, substrate stiffness determined growth patterns of Xenopus retinal ganglion cell axons. In vivo atomic force microscopy revealed a noticeable pattern of stiffness gradients in the embryonic brain. Retinal ganglion cell axons grew toward softer tissue, which was reproduced in vitro in the absence of chemical gradients. To test the importance of mechanical signals for axon growth in vivo, we altered brain stiffness, blocked mechanotransduction pharmacologically and knocked down the mechanosensitive ion channel piezo1. All treatments resulted in aberrant axonal growth and pathfinding errors, suggesting that local tissue stiffness, read out by mechanosensitive ion channels, is critically involved in instructing neuronal growth in vivo.
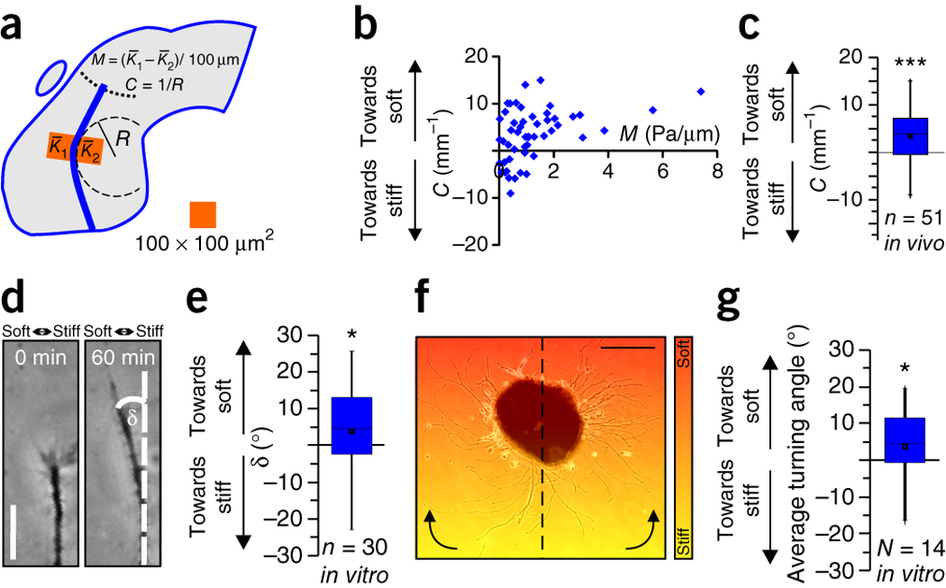
Figure 3: Neurons grow toward soft tissue. (a) Schematic showing how local gradients in brain tissue stiffness perpendicular to the RGC axon growth direction M and the local OT curvature C were determined. (b) Relationship between M and C. (c) Same data as in b, pooled. Axons in vivo preferentially turned toward the softer side of the tissue (one-sample Wilcoxon signed-rank test; P = 1.44 × 10−4, z = 3.801). n = number of measurement points from 7 animals. (d,e) Time-lapse imaging of individual axon bundles growing on a stiffness gradient matching that found in vivo (Mmax = ~2 Pa/μm; Supplementary Fig. 1) revealed that in vitro, in the absence of chemical gradients, RGC axons preferentially turned toward the softer side of the substrate (one-sample Wilcoxon signed-rank test; P = 0.0549; z = −1.913). Scale bar: 20 μm. Representative images from 11 biological replicates are shown. (f) Eye primordium cultured on a similar stiffness gradient (indicated by color). Axons growing more clockwise in the left half and more counterclockwise in the right half of the image turned toward the soft side of the substrate. Scale bar: 200 μm. The experiment was repeated three times, and a representative image is shown. (g) Quantification of individual axon segment orientations similarly revealed preferential turning toward the soft side of the substrate (one-sample Wilcoxon signed-rank test; P = 0.0264, z = 2.197). Boxes show the 25th, 50th (median), and 75th percentiles; whiskers show the spread of the data (excluding outliers), and an 'O' the mean score for a group. *P ≤ 0.05; ***P = 1.44 × 10−4.
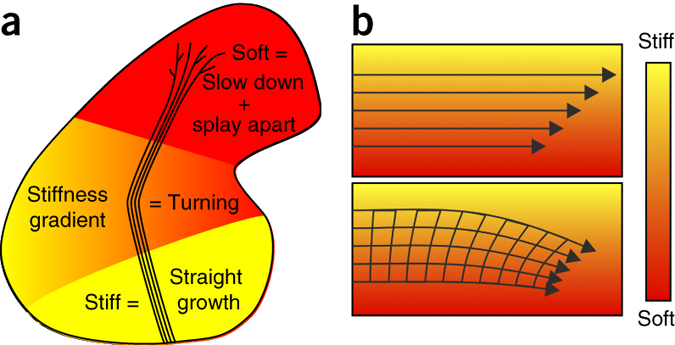
Figure 6: Schematics of the mechanical control of axon growth. (a) Schematic summary of this study. Shown is the outline of a Xenopus brain and the OT. Mechanical signals contribute to neuronal growth in the developing CNS. RGC axons grow faster, straighter, and more parallel on stiffer than on softer substrates (Fig. 1). Accordingly, the part of the brain these axons have to pass is stiffer than the tectum (Fig. 2), where axon growth slows down and eventually stops. The softness of the tectum then facilitates unbundling and branching. On their way, axons encounter an area with a stiffness gradient, which contributes to the turning of the OT toward the softer side of the brain (Fig. 3). (b) Schematic illustration of a mechanism by which axon bundles encountering a perpendicular stiffness gradient might turn toward the softer side of the substrate. The velocity of axons is larger on stiffer substrates (upper panel). As axons in the OT fasciculate, they are mechanically coupled, so the faster axons growing on the stiffer side may be pulled toward the slower axons on the softer side, leading to a reorientation of the axon bundle and overall growth toward the softer side.
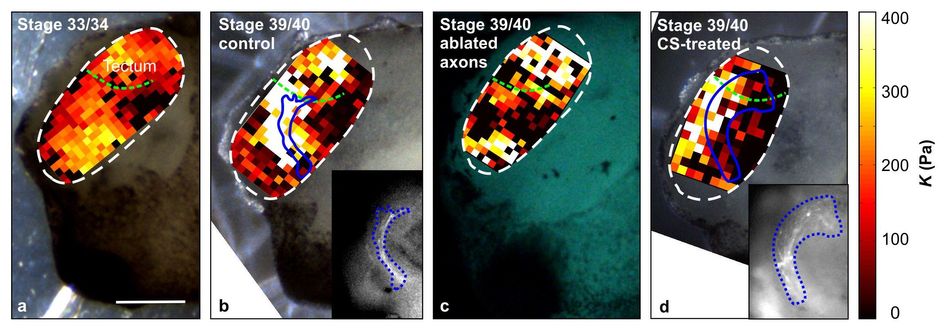
Supplementary Figure 3: Elasticity maps for a fixed indentation depth. (68 KB) (a, b, d) Same samples as shown in Figs. 2c, d, and 4a, respectively; here K was determined not at a defined force but at a defined indentation depth δ = 3 µm. Stiffness gradients in the tissue are very similar to those shown in Figs. 2 and 4. Blue lines indicate outlines of the OT. Insets in (b) and (d): epifluorescence images of RGC axons in the OT of the respective brains. (c) Stiffness map of a brain with ablated RGC axons. Similar stiffness gradients are found as in control brains, indicating that gradients likely arise from the distribution of cell somata in the tissue (cf. Fig. 2e). Scale bar: 200 µm. Experiments were repeated three times and representative images are shown.
Reprinted by permission from Macmillan Publishers Ltd on behalf of Nature Neuroscience, copyright 2016.
Last Updated: 2016-11-16
