Asymmetric cell division breaks pluripotency in vivo
Elife. March 3, 2017; 6
Lineage commitment of embryonic cells involves MEK1-dependent clearance of pluripotency regulator Ventx2
Scerbo P, Marchal L, Kodjabachian L.
How embryonic pluripotent cells can maintain an uncommitted state as well as an unrestricted potential for multi-lineage commitment is a key and unresolved question in developmental and stem cell biology. Using Xenopus laevis, Scerbo P et al. have demonstrated the importance of asymmetric cell division (ACD) and developmentally-regulated proteolysis for the transition of embryonic cells from pluripotent to committed states. Using confocal imaging, they demonstrated that the pluripotency factor Ventx2 is asymmetrically distributed in dividing pluripotent embryonic cells. This process is under the control of the kinase MEK1, which is required for asymmetric degradation of Ventx2 during mitosis of pluripotent cells, as well as for global clearance of Ventx2 at gastrulation. In absence of MEK1 activity, Ventx2 protein is stabilized and symmetrically distributed in pluripotent cells. Ventx2 stabilization causes embryonic cells to lose their competence to differentiate in response to lineage inducers, and to instead maintain high expression of the zygotic pluripotency genes Pou5f3.2/Oct4 (Oct25) and Pou5f3.1/Oct4 (Oct91). MEK1-deficient cells exit pluripotency and resume differentiation, when Ventx2 is also knocked down. Although many studies described the relevance of ACD in cell fate instruction, mainly using invertebrate models, this study is the first to demonstrate that vertebrate pluripotent cells divide asymmetrically in vivo. Ventx2 represents the first pluripotency factor shown to be asymmetrically inherited during embryonic cell division. Considering that human VENTX is one of the most specific marker of naïve pluripotency in human epiblast cells (PMID:28953884; PMID:29367672), our study further supports the use of Xenopus as a relevant model to identify conserved fundamental principles of pluripotency.
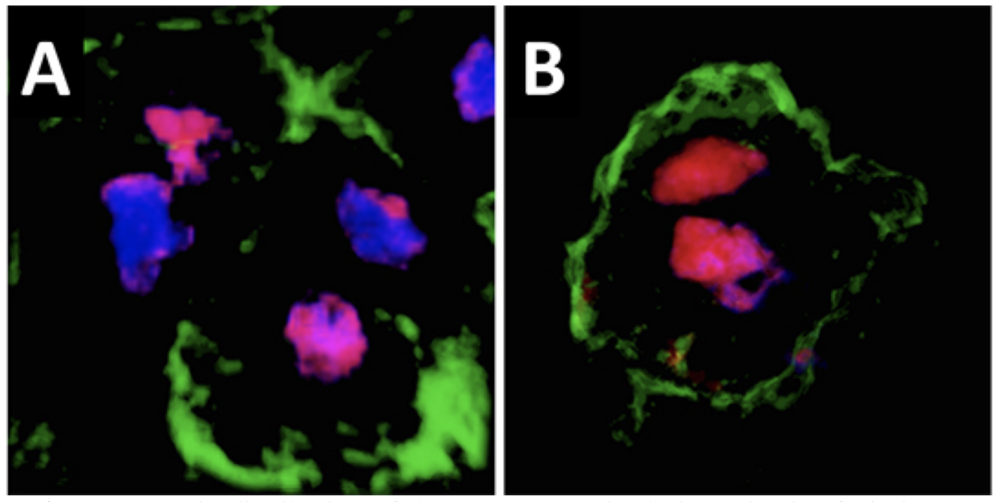
Asymmetric distribution of the Ventx2 protein during mitosis of pluripotent cells. A. In a control embryo, the Ventx2 protein (magenta) is unequally distributed between the two daughter nuclei (blue) of dividing pluripotent cells. B. A form of Ventx2 resistant to degradation induced by MEK1 is equally distributed between the two daughter nuclei. Cell membranes are identified by the presence of GFP signal (green).
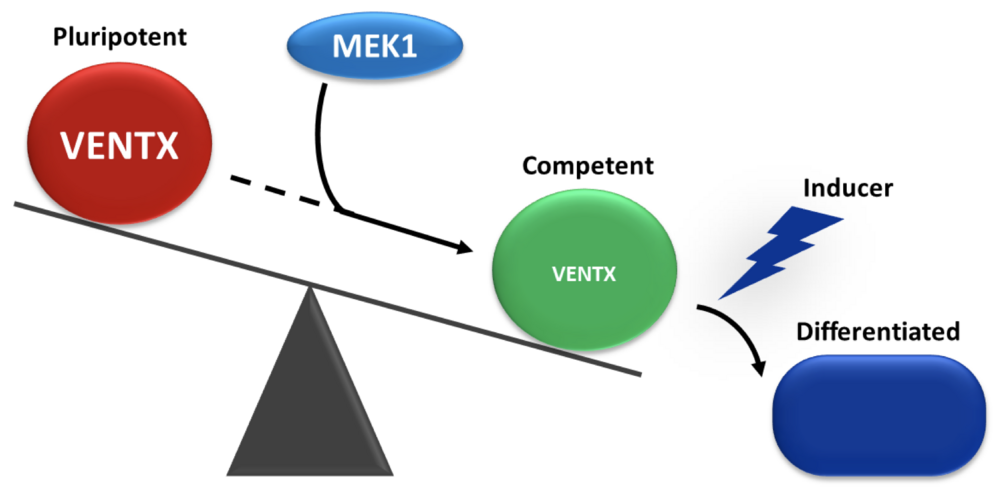
Balance between pluripotency and commitment in vivo. In the young embryo, cells switch from a pluripotent state to a state of competence to respond to differentiating inducing signals. This transition requires the action of the MEK1 kinase, which causes the disappearance of the Ventx2 pluripotency factor.
Click here to view article at Elife.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
During early embryogenesis, cells must exit pluripotency and commit to multiple lineages in all germ-layers. How this transition is operated in vivo is poorly understood. Here, we report that MEK1 and the Nanog-related transcription factor Ventx2 coordinate this transition. MEK1 was required to make Xenopus pluripotent cells competent to respond to all cell fate inducers tested. Importantly, MEK1 activity was necessary to clear the pluripotency protein Ventx2 at the onset of gastrulation. Thus, concomitant MEK1 and Ventx2 knockdown restored the competence of embryonic cells to differentiate. Strikingly, MEK1 appeared to control the asymmetric inheritance of Ventx2 protein following cell division. Consistently, when Ventx2 lacked a functional PEST-destruction motif, it was stabilized, displayed symmetric distribution during cell division and could efficiently maintain pluripotency gene expression over time. We suggest that asymmetric clearance of pluripotency regulators may represent an important mechanism to ensure the progressive assembly of primitive embryonic tissues.
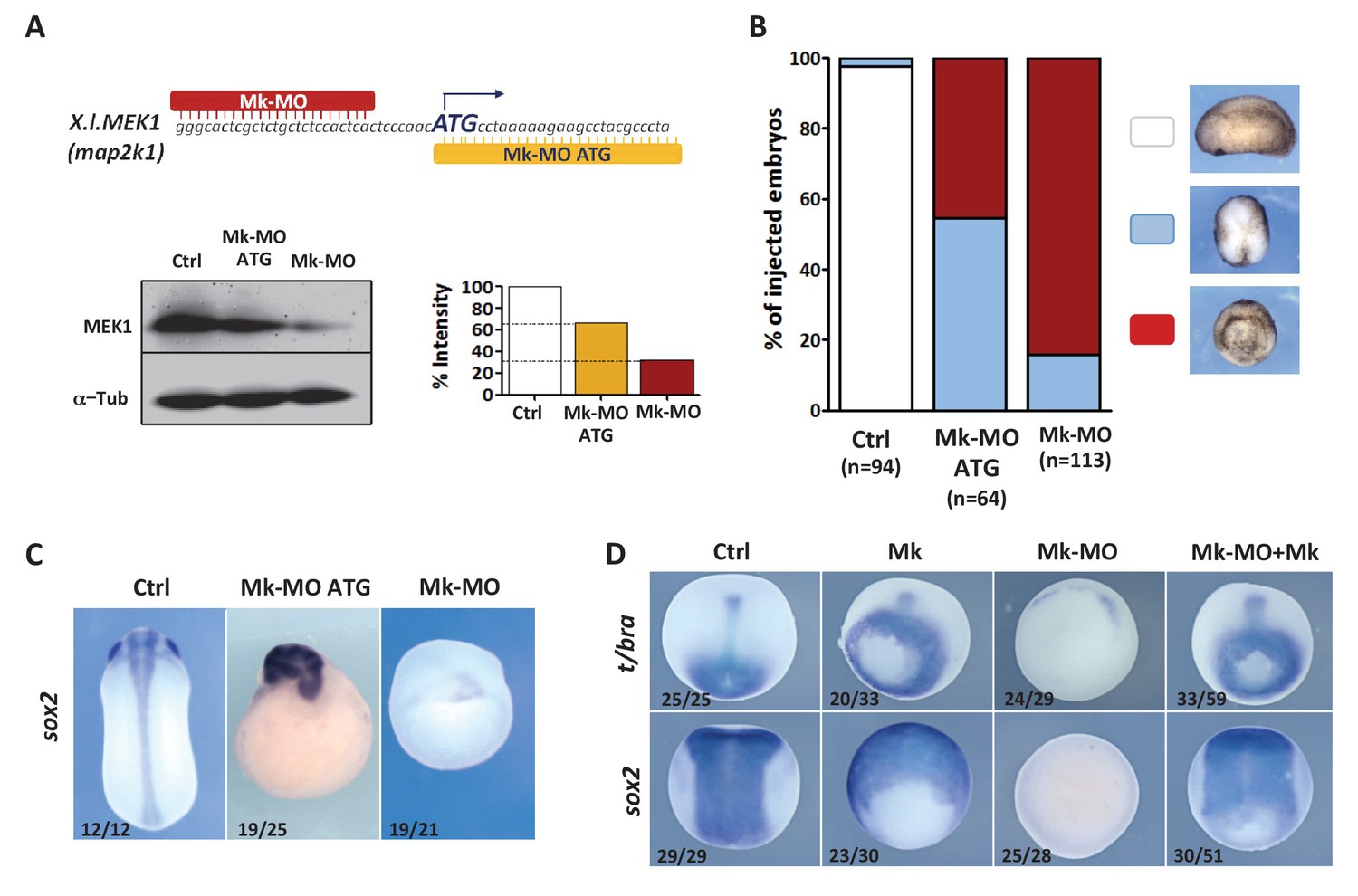
Figure 1. MEK1 depletion impairs embryonic development. (A) Mk-MO and Mk-MO ATG were designed to target MEK1 translation. Western blot analysis of blastula stage nine embryos injected with 25 ng per blastomere of either MO at the 4 cell stage revealed reduced MEK1 translation. α-Tubulin was used as a loading control. Control embryos were uninjected. The histogram shows the normalized intensity of MEK1 signals relative to control. (B) Embryos were injected as in (A) and morphology was analyzed at tailbud stage. (C) Embryos injected as in (A) were stained with Sox2 probe to highlight defective axis formation and neural tissue differentiation. (D) Embryos were injected at the 2 cell stage with 25 ng Mk-MO per cell, and at the 4- cell stage with 400 pg of mammalian MEK1 (Mk) RNA per cell and processed for WISH analysis at late gastrula stage 13 with t/bra probe to highlight the mesoderm (dorso-vegetal view) and with sox2 to highlight the neurectoderm (dorsal view). In C and D, the number of embryos exemplified by the photograph over the total number of embryos analyzed is indicated.
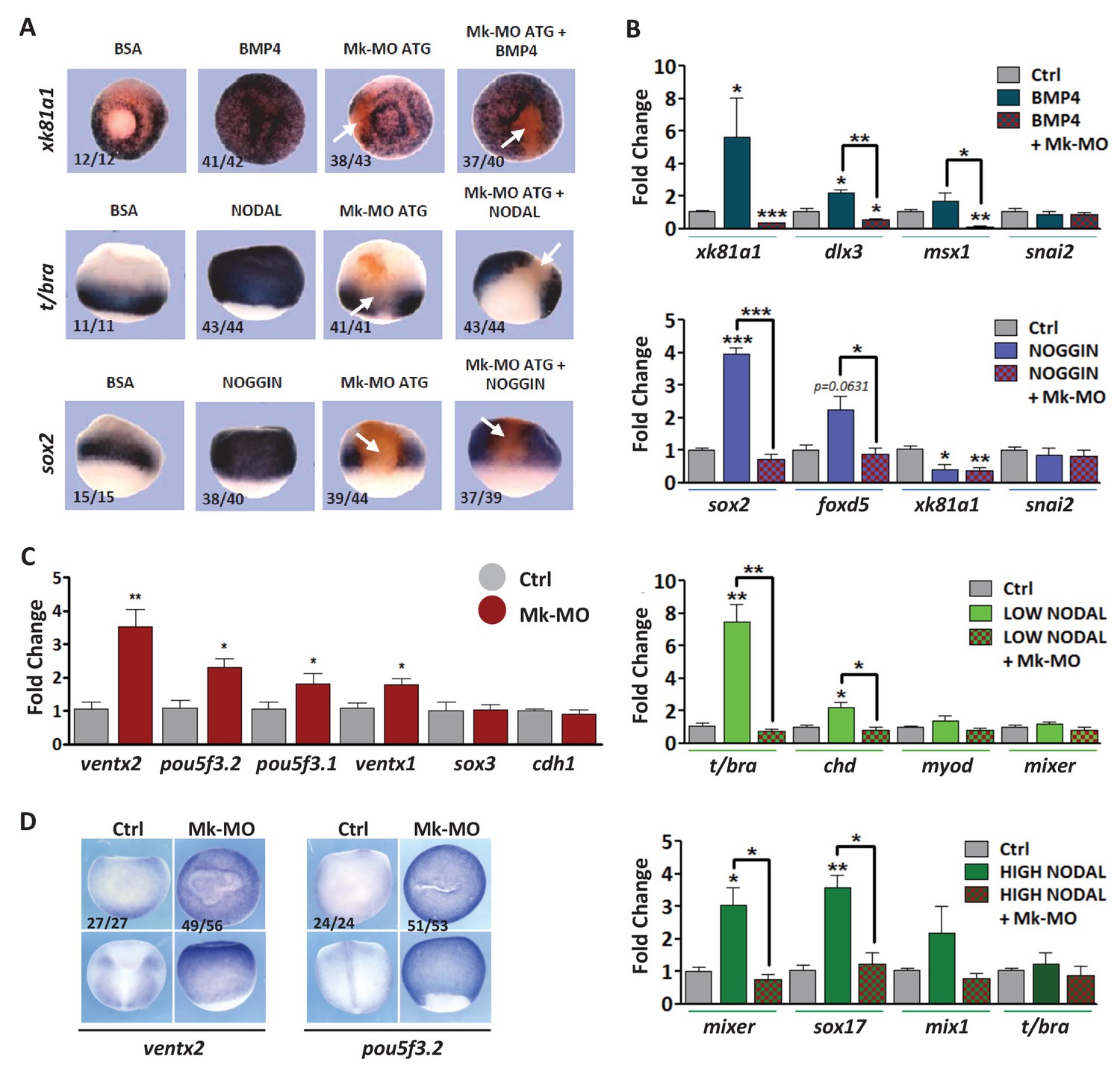
Figure 2. MEK1 depletion affects cell competence to exit pluripotency and enter into differentiation. (A) Sixteen-cell embryos were injected in one animal blastomere with 25 ng Mk-MO ATG and 2.5 ng FLDX. Next, these embryos were injected at blastula stage 8.5 with recombinant BMP4 (2 ng), NODAL (10 ng), or NOGGIN (36 ng) proteins into the blastocoele, collected at early gastrula stage 10.5, and processed for WISH with xk81a1 (epidermis, animal view), t/bra (mesoderm, lateral view), and sox2 (neural tissue, dorsal view). FLDX (orange staining) was used to trace Mk-MO injected cells (white arrows). (B) Four-cell embryos were injected with 25 ng Mk-MO per blastomere, animal caps were explanted at blastula stage 8.5 and cultured in the presence of 20 ng/ml BMP4, 100 ng/ml NOGGIN, 20 ng/ml NODAL (low), or 200 ng/ml NODAL (high) until late gastrula stage 13, and processed for RT-qPCR. (C) Four-cell embryos were injected with 25 ng Mk-MO per blastomere, collected at stage 10.5 and processed for RT-qPCR. (D) Embryos injected as in (C) were processed for WISH analysis at late gastrula stage 13 with pou5f3.2 (oct25) and ventx2 probes. a: animal view; v: ventral view; l: lateral view; d: dorsal view. For all qPCR graphs, error bars represent s.e.m. values of four independent experiments with two technical duplicates. For statistical analyses, samples were compared with the respective control by Unpaired Student’s t-test. *p<0.05, **p<0.005. ***p<0.0001. In A and D, the number of embryos exemplified by the photograph over the total number of embryos analyzed is indicated.
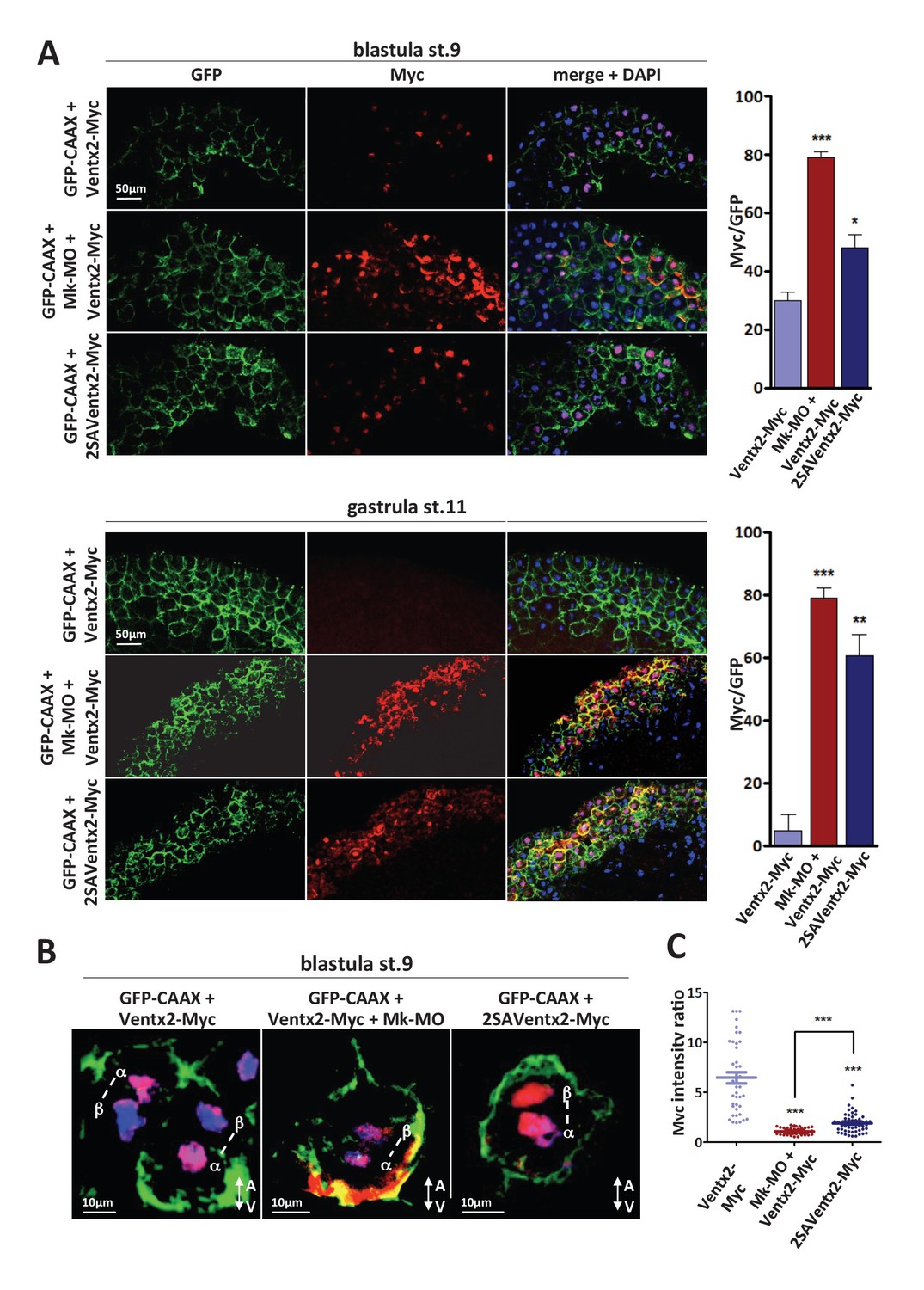
Figure 3. MEK1 is required for Ventx2 clearance and asymmetric distribution during cell division. (A,B) Four-cell embryos were injected in each cell with 50 pg GFP-CAAX, 50 pg Ventx2-Myc, 50 pg 2SAVentx2-Myc RNAs, and 25 ng Mk-MO, as indicated. Embryos were fixed at blastula stage 9, or gastrula stage 11, cryosectioned and processed for anti-Myc (red), and anti-GFP (green) immunostaining, and DNA was stained with DAPI (blue). Graphs show the percentage of Myc positive nuclei (DAPI positive) over the total number of injected cells (GFP positive) from four independent experiments. (B) 3D reconstruction of confocal slices of mitotic Myc positive nuclei labeled by DAPI from stage nine sectioned embryos. Sister mitotic chromosomes are referred to as α (more intense Myc staining), and β (less intense Myc staining). The A-V arrows indicate the animal-vegetal axis. Note the asymmetric cortical Ventx2-Myc signal in the MEK1 morphant cell. (C). The graph shows the ratios of Myc signal intensity betweenα and β sister nuclei.
Adapted with permission from eLife Publications Ltd: Scerbo et al. (2017). Lineage commitment of embryonic cells involves MEK1-dependent clearance of pluripotency regulator Ventx2. Elife. March 3, 2017; 6, copyright (2017).
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2018-03-08
