RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6
Nature. May 16, 2018;
Szenker-Ravi E, Altunoglu U, Leushacke M, Bosso-Lefèvre C, Khatoo M, Thi Tran H, Naert T, Noelanders R, Hajamohideen A, Beneteau C, de Sousa SB, Karaman B, Latypova X, Baaran S, Yücel EB, Tan TT, Vlaeminck L, Nayak SS, Shukla A, Girisha KM, Le Caignec C, Soshnikova N, Uyguner ZO, Vleminckx K, Barker N, Kayserili H, Reversade B.
Click here to view article in Nature.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
The four R-spondin secreted ligands (RSPO1-RSPO4) act via their cognate LGR4, LGR5 and LGR6 receptors to amplify WNT signalling1-3. Here we report an allelic series of recessive RSPO2 mutations in humans that cause tetra-amelia syndrome, which is characterized by lung aplasia and a total absence of the four limbs. Functional studies revealed impaired binding to the LGR4/5/6 receptors and the RNF43 and ZNRF3 transmembrane ligases, and reduced WNT potentiation, which correlated with allele severity. Unexpectedly, however, the triple and ubiquitous knockout of Lgr4, Lgr5 and Lgr6 in mice did not recapitulate the known Rspo2 or Rspo3 loss-of-function phenotypes. Moreover, endogenous depletion or addition of exogenous RSPO2 or RSPO3 in triple-knockout Lgr4/5/6 cells could still affect WNT responsiveness. Instead, we found that the concurrent deletion of rnf43 and znrf3 in Xenopus embryos was sufficient to trigger the outgrowth of supernumerary limbs. Our results establish that RSPO2, without the LGR4/5/6 receptors, serves as a direct antagonistic ligand to RNF43 and ZNRF3, which together constitute a master switch that governs limb specification. These findings have direct implications for regenerative medicine and WNT-associated cancers.
Slider photo of frog mutant - Amelie Fossul.
The slider item photo shows a mosaic mutant Xenopus tropicalis injected on its right site with TALENs targeting znrf3 and rnf43 (resulting in duplication of the fore limb) while its left side was injected with a CRISPR guide RNA targeting rspo2 (which resulted in the absence of the fore limb).
This study is a collaboration between the Reversade Lab (Laboratory of Human Embryology and Genetics, A*STAR, Singapore) & Vleminckx Lab (Department of Biomedical Molecular Biology & Center for Medical Genetics, Ghent University, Belguim). This highlights the power of utilizing the Xenopus tropicalis model organism system to study human disease.
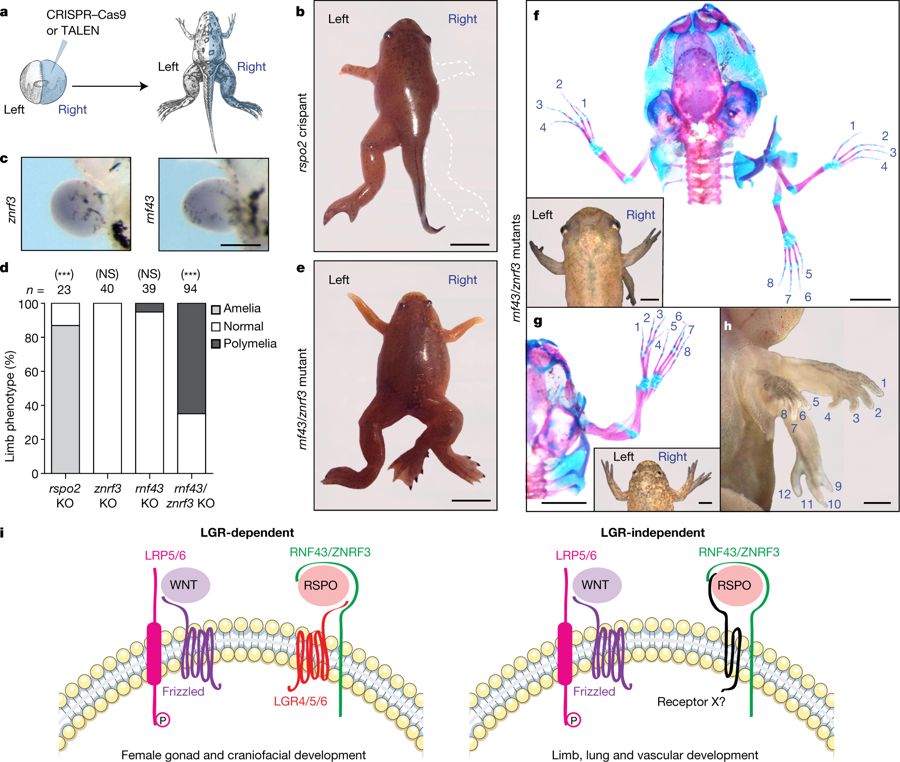
Fig. 4: Frogs mutant for both rnf43 and znrf3 display complete limb duplications.
a, Experimental scheme using X. tropicalis. b, Representative rspo2 crispant (stage 63; n = 21). Scale bar, 0.5 cm. c, znrf3 and rnf43 in situ hybridization in stage 50 limb buds. Scale bar, 300 μm. The experiment was repeated three times. d, Scoring of limb phenotype. n denotes number of froglets. NS, not significant; ***P < 0.001, significantly different from normal (χ2 test). e, Stage 67 znrf3/rnf43 double mutant with a duplicated right hindlimb. Scale bar, 0.5 cm. f, g, External view and alizarin red and alcian blue staining of rnf43/znrf3 double mutants (stages 62 (f) and 66 (g)). Scale bars, 0.3 cm. h, rnf43/znrf3 double-mutant tadpole (stage 59) displaying quadruplication of the right forelimb (three are visible). Scale bar, 0.2 cm. In e–h, 61 znrf3/rnf43 double-mutant froglets with polymelia were obtained. i, Updated model for LGR-dependent R-spondin processes (left), and LGR-independent RSPO2/3 signalling (right), which may involve the activity of a hitherto unknown receptor X.
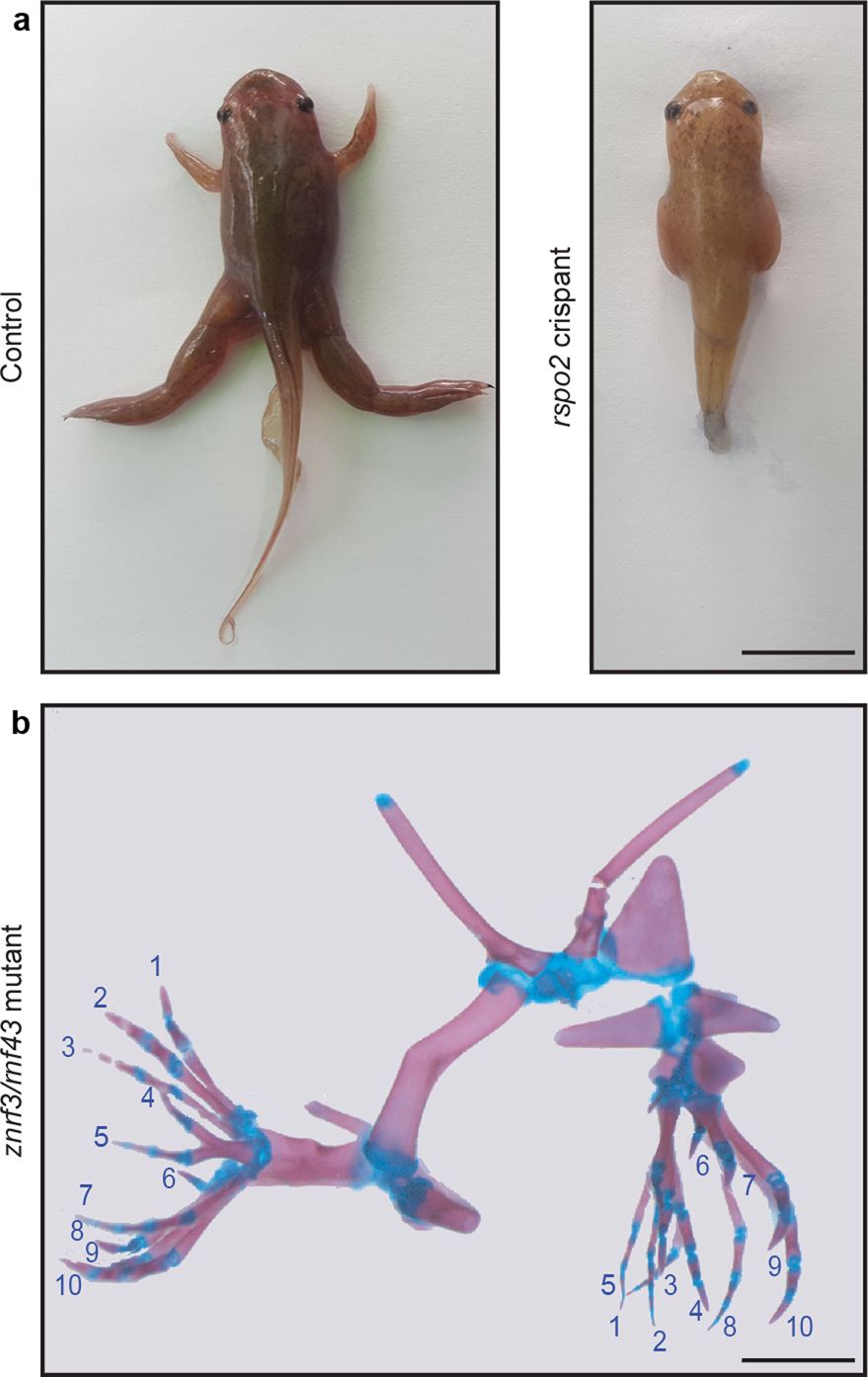
Extended Data Fig. 7 Extreme phenotypes of Xenopus tropicalis mutant tadpoles.
a, Control and rspo2 CRISPR–Cas9-injected tadpole showing a complete tetra-amelia phenotype probably due to incomplete cleavage at the time of injection and leakage of the CRISPR–Cas9 between the two blastomeres (n = 3 froglets). Scale bar, 1 cm. b, Alizarin red and alcian blue staining of a double-mutant rnf43/znrf3 TALEN-injected tadpole showing complete mirror-image diplopodia of both hindlimbs with 10 digits each (n = 4 froglets). Scale bar, 0.2 cm.
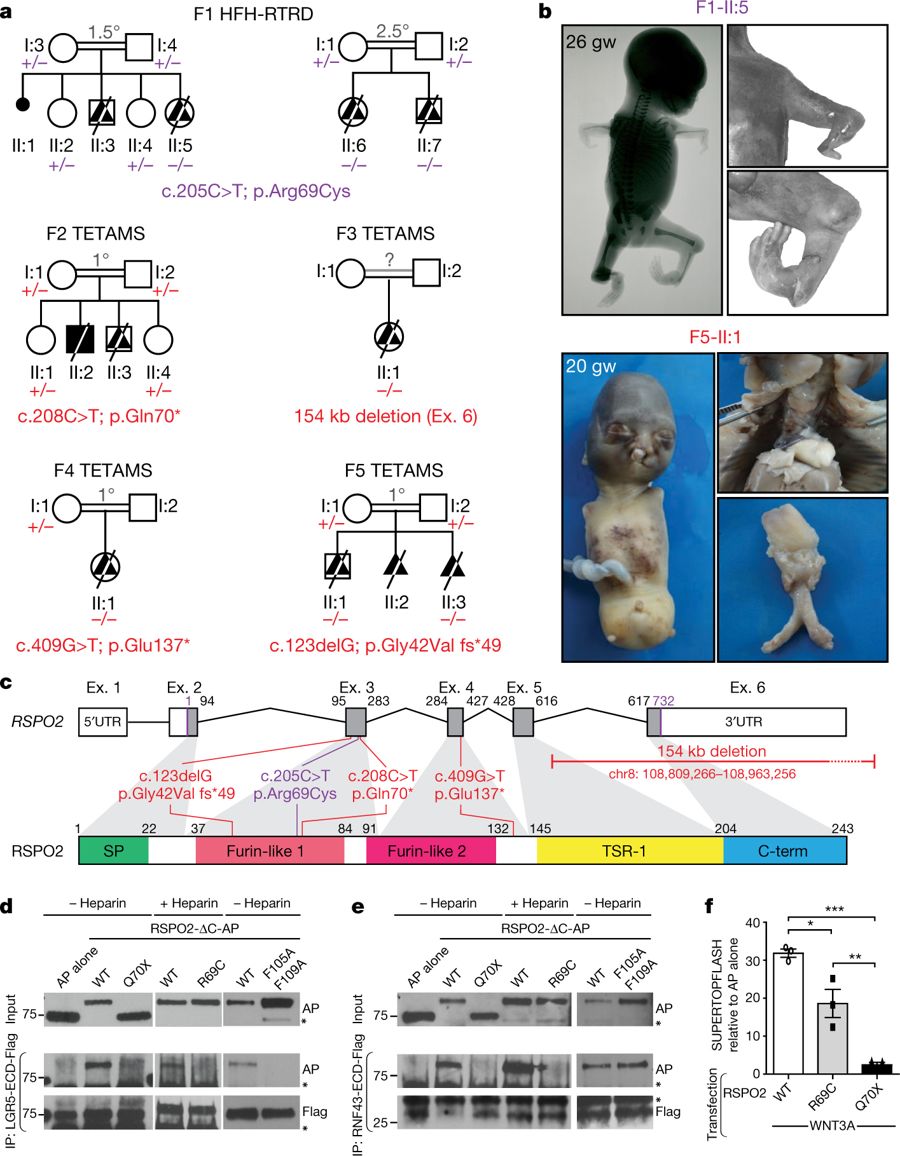
Fig. 1: Identification of RSPO2 mutations in fetuses presenting with severe limb defects.
a, Pedigrees of family 1 (F1) with HFH-RTRD and families 2–5 (F2–F5) with TETAMS, and RSPO2 germline mutation status for available family members. b, Pictures and radiographs illustrating limb defects in a fetus with HFH-RTRD, and complete absence of limbs and lungs in a fetus with TETAMS. gw, gestational weeks. c, RSPO2 genomic (top) and protein (bottom) structures with identified mutations. Ex., exon. UTR, untranslated region. d, Co-immunoprecipitation (IP) of alkaline phosphatase (AP)-tagged RSPO2 lacking the C-terminal domain (ΔC; RSPO2-ΔC-AP) with Flag-tagged extracellular domains (ECD) of LGR5 (LGR5-ECD-Flag). WT, wild-type. e, Co-immunoprecipitation of RSPO2-ΔC-AP with RNF43-ECD-Flag. Asterisks indicate non-specific bands. Experiments in d and e were repeated three times. f, SUPERTOPFLASH assay in HEK293T-STF cells transfected with WNT3A and the indicated RSPO2 constructs. n = 3 biological replicates. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) with Bonferroni’s correction. For gel source data, see Supplementary Fig. 1.
Adapted with permission from Macmillan Publishers Ltd: Szenker-Ravi et al. (2018). RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Naturevolume 557, pages564–569 (2018)
doi:10.1038/s41586-018-0118-y, copyright (2018).
