Lrp2-mediated endocytosis is essential for neural tube closure
Neural tube closure requires the endocytic receptor Lrp2 and its functional interaction with intracellular scaffolds
Izabela Kowalczyk, Chanjae Lee, Elisabeth Schuster, Josefine Hoeren, Valentina Trivigno, Levin Riedel, Jessica Görne, John B. Wallingford, Annette Hammes, Kerstin Feistel
Development 2021 148: dev195008 doi: 10.1242/dev.195008 Published 26 January 2021
Click here to view article at Development.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
ABSTRACT
Pathogenic mutations in the endocytic receptor LRP2 in humans are associated with severe neural tube closure defects (NTDs) such as anencephaly and spina bifida. Here, we have combined analysis of neural tube closure in mouse and in the African Clawed Frog Xenopus laevis to elucidate the etiology of Lrp2-related NTDs. Lrp2 loss of function impaired neuroepithelial morphogenesis, culminating in NTDs that impeded anterior neural plate folding and neural tube closure in both model organisms. Loss of Lrp2 severely affected apical constriction as well as proper localization of the core planar cell polarity (PCP) protein Vangl2, demonstrating a highly conserved role of the receptor in these processes, which are essential for neural tube formation. In addition, we identified a novel functional interaction of Lrp2 with the intracellular adaptor proteins Shroom3 and Gipc1 in the developing forebrain. Our data suggest that, during neurulation, motifs within the intracellular domain of Lrp2 function as a hub that orchestrates endocytic membrane removal for efficient apical constriction, as well as PCP component trafficking in a temporospatial manner.
Authors' summary:
Pathogenic variants of the endocytic receptor LRP2 are associated with severe forebrain defects in humans. Comparing Xenopus and mouse anterior neurulation, Kowalczyk et al. identify a conserved role for Lrp2 in anterior neural tube closure and forebrain development. Their study highlights the importance of Lrp2-mediated endocytosis for neural morphogenesis, as loss of Lrp2 affects both apical constriction (AC) as well as planar cell polarity (PCP). They show that the receptor functionally interacts with intracellular adaptors, suggesting that Lrp2 acts as a hub to orchestrate AC and PCP in a temporospatial manner - a prerequisite for correct anterior neural tube closure.
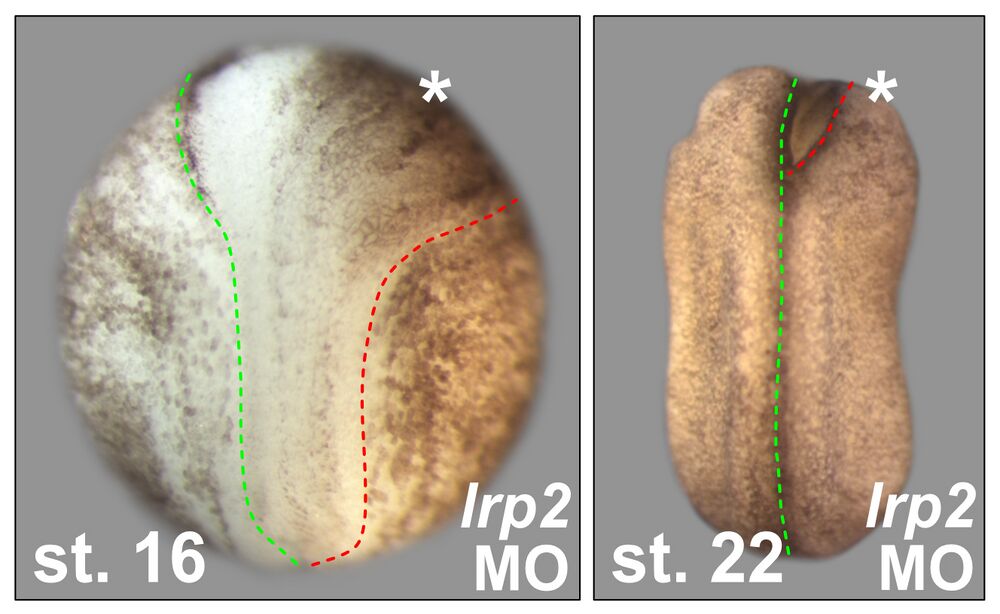
Neural tube closure defects:
Lrp2 is required for neural tube closure. 8-cell embryos were injected into one of the dorsal animal blastomeres to target the neural plate unilaterally (asterisks). Morpholino oligomer (MO) for lrp2 impaired hingepoint formation and neural plate narrowing during neural fold elevation stages (st. 16), culminating in an open anterior neural fold on the injected side even after neural tube closure occurs in WT (st. 22). Green and red dashed lines indicate the neural plate border on the uninjected and injected side, respectively.
Attribution: Kerstin Feistel
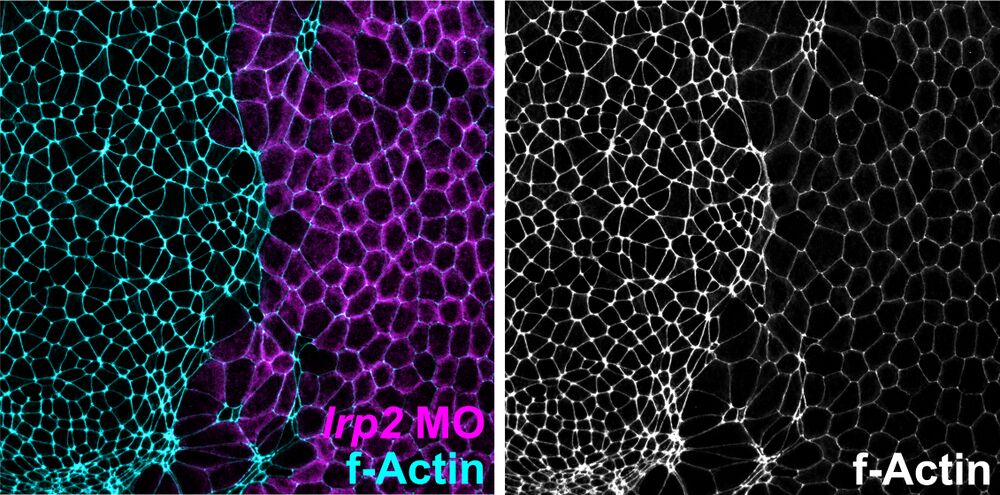
Apical constriction defects:
Loss of Lrp2 impairs apical constriction of neuroepithelial cells. Analysis of apical constriction in Xenopus neural plate cells. f-Actin revealed a larger apical cell surface in cells injected with lrp2 morpholino oligomer (MO; identified by lineage tracer, magenta) as compared to uninjected side.
Attribution: Kerstin Feistel
Published with the authors' permission.
