The Wnt/PCP formin Daam1 drives cell-cell adhesion during nephron development
Vanja Krneta-Stankic, Mark E. Corkins, Adriana Paulucci-Holthauzen, Malgorzata Kloc, Andrew B. Gladden, Rachel K. Miller
Cell Reports, Vol. 36, Issue 1, 109340, JULY 06, 2021
DOI:https://doi.org/10.1016/j.celrep.2021.109340
Click here to view article at Cell Reports.
Click here to view article at Pubmed.
In brief
How cells remodel their adhesions through cell-surface proteins such as E- cadherin is a central question in epithelial tissue biology. Krneta-Stankic et al. show that the Wnt/PCP formin Daam1 regulates cytoskeletal membrane dynamics and E-cadherin localization within developing nephrons. These findings provide a new framework for studying cell-cell adhesion and nephron morphogenesis.
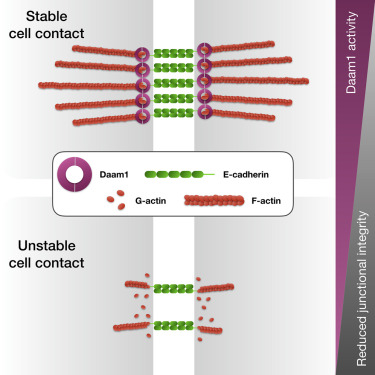
Highlights
• Daam1 localizes to cell-cell contacts in developing nephron
• Daam1 regulates the cytoskeletal membrane dynamics
• Daam1 promotes E-cadherin localization
• Formin homology domain 2 (FH2) of Daam1 mediates E-cadherin localization
Abstract
E-cadherin junctions facilitate assembly and disassembly of cell contacts that drive development and homeostasis of epithelial tissues. In this study, using Xenopus embryonic kidney and Madin-Darby canine kidney (MDCK) cells, we investigate the role of the Wnt/planar cell polarity (PCP) formin Daam1 (Dishevelled-associated activator of morphogenesis 1) in regulating E-cadherin-based intercellular adhesion. Using live imaging, we show that Daam1 localizes to newly formed cell contacts in the developing nephron. Furthermore, analyses of junctional filamentous actin (F-actin) upon Daam1 depletion indicate decreased microfilament localization and slowed turnover. We also show that Daam1 is necessary for efficient and timely localization of junctional E-cadherin, mediated by Daam1’s formin homology domain 2 (FH2). Finally, we establish that Daam1 signaling promotes organized movement of renal cells. This study demonstrates that Daam1 formin junctional activity is critical for epithelial tissue organization.
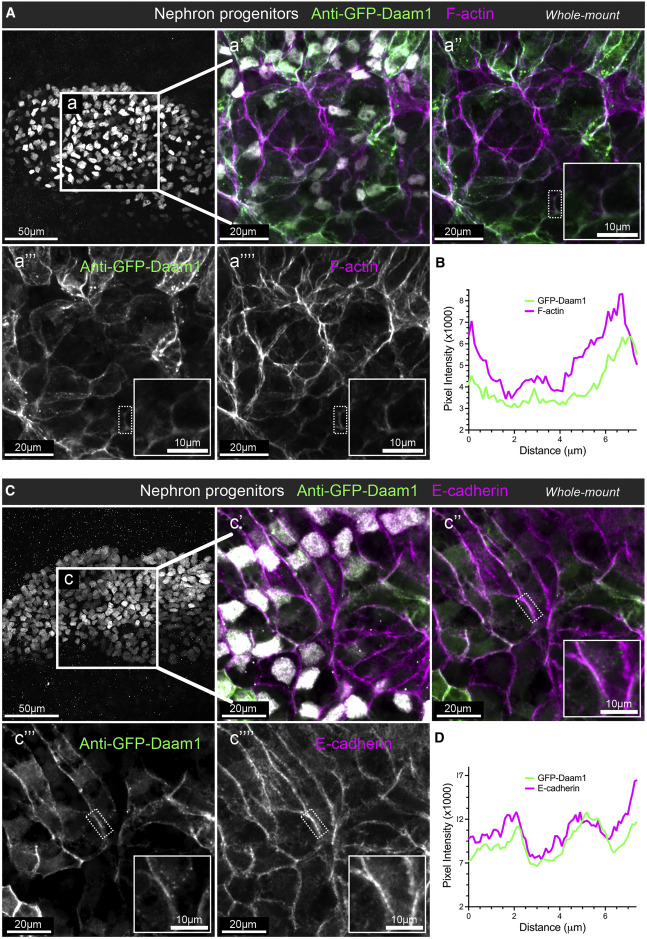
Figure 1. Daam1 co-localizes with junctional F-actin and E-cadherin during early nephron development
(A) Confocal maximum image projections of whole-mount immunostaining of Xenopus nephric primordium labeled by Lhx1 (white) and GFP to visualize Daam1 (green) in conjunction with phalloidin staining to visualize F-actin (magenta); scale bar, 50 mm. a’–a’’’’ are close-up images of region a; scale bars, 20 mm. The first panel consists of the entire z stack to show the cell positions within the entire kidney. In contrast, images displayed in a’–a’’’’ contain a subset of the z slices to exclude the intense signal from phalloidin within the Xenopus skin. The white dotted box in a’’–a’’’’ marks the junction shown enlarged in the insets (scale bars, 10 mm) and analyzed in (B).
(B) A line plot showing the junctional intensity of F-actin (magenta) and GFP-Daam1 (green) of the junction highlighted in (A), a’’–a’’’’, by the white dotted box. (C) Confocal maximum image projections of whole-mount immunostaining of Xenopus nephric primordium labeled by Lhx1 (white) and GFP to visualize Daam1 (green) in conjunction with E-cadherin (magenta); scale bar, 50 mm. c’–c’’’’ are close-up images of region c; scale bars, 20 mm. The first panel consists of the entire z stack to show the cell positions within the entire kidney. In contrast, images displayed in c’–c’’’’ contain a subset of the z slices to exclude the intense signal from E-cadherin within the Xenopus skin. The white dotted box in c’’–c’’’’ marks the junction shown enlarged in the insets (scale bars, 10 mm) and analyzed in (D). (D) A line plot showing the junctional intensity of E-cadherin (magenta) and GFP-Daam1 (green) of the junction highlighted in c’’–c’’’’ by the white dotted box.
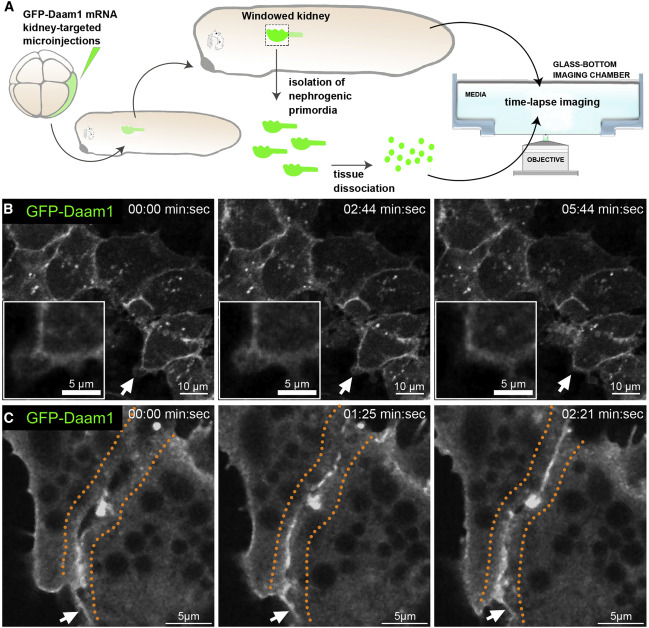
Figure 2. Daam1 localizes to newly formed cell-cell contacts
(A) Schematic showing the steps involved in preparing ‘‘windowed kidney’’ embryos and primary cultures expressing GFP-Daam1. For clarity, the 8-cell GFP- Daam1-injected Xenopus blastomere is fate-mapped strictly to the nephric primordium, and blastomere also contributes to the epidermis, ventral and dorsal somites, hindgut, proctodeum, and trunk neural crest cells (DeLay et al., 2016; Moody and Kline, 1990).
(B) Time-lapse imaging montage of the nephric primordium expressing GFP-Daam1 in ‘‘windowed kidney’’ embryos. Elapsed time is indicated at the top; scale bars, 10 mm. The white arrows point to cellular protrusions shown enlarged in the insets (scale bars, 5 mm); see Video S1.
(C) Time-lapse imaging montage showing cells isolated from a developing nephron expressing GFP-Daam1 mRNA adhering to each other. Elapsed time is indicated at the top; scale bars, 5 mm. The orange dotted line delineates the border arising between two cells, and the white arrows point to filopodium-like cellular protrusions; see Video S2.
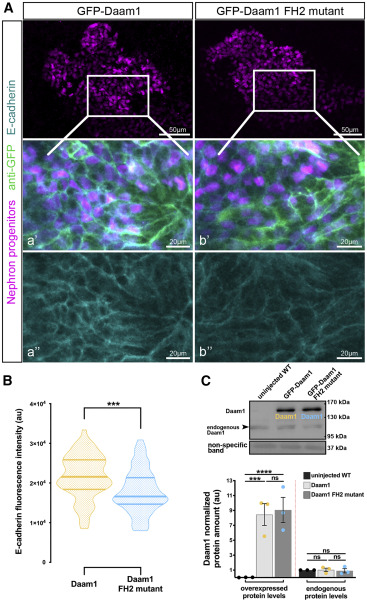
Figure 6. The FH2 domain of Daam1 mediates E-cadherin localiza- tion to cell junctions
(A) Maximum-projection confocal images of whole-mount immunostaining showing E-cadherin (cyan) in Xenopus nephric primordium labeled by Lhx1 (magenta) expressing GFP-Daam1 or GFP-Daam1 FH2 mutant mRNA (labeled by anti-GFP, green); scale bars, 50 mm. a’–a’’ and b’–b’’ are close-up images of the corresponding regions in the white boxes; scale bars, 20 mm. The top panels consist of the entire z stacks to show nephric progenitors’ cell positions within the developing kidney. In contrast, images displayed in a–a’ and b–b’ contain a subset of the z slices to exclude the intense signal from E-cadherin expressed in the epithelium of the Xenopus skin. a’–b’, nephric cell progenitors labeled by Lhx1 (magenta), anti-GFP (green), and E-cadherin (cyan); a’’–b’’, E- cadherin (cyan).
(B) Violin plots depicting the relative fluorescence intensity of junctional E- cadherin in nephric primordia expressing GFP-Daam1 (orange) and GFP- Daam1 FH2 mutant (blue) mRNA. nDaam1 = 60 junctions on 3 embryos and nDaam1FH2mutant = 55 junctions on 3 embryos. Centerlines show the median; limits show the first and third quartiles. ***p % 0.001, analyzed by unpaired t test.
(C) Western blot and the graph of densitometry measures showing the exog- enous and endogenous protein levels of Daam1 in uninjected WT embryos and embryos injected with 1 ng Daam1 or Daam1 FH2 mutant mRNA. The non- specific band confirms equal loading. Embryo lysates were pooled from 10–20 1-cell injected embryos at stages NF 11–12, and approximately 1/2 embryo per lane. The results are expressed as means ± SEM from three independent experiments. Individual band intensities are normalized to the uninjected band and plotted in a.u. for uninjected (black), Daam1 (orange), and Daam1 FH2 mutant (blue). nsp > 0.05, ***p % 0.001, ****p % 0.0001, analyzed by one-way ANOVA.
Adapted with permission from Cell Press on behalf of Cell Reports: Krneta-Stankic et al. (2021). The Wnt/PCP formin Daam1 drives cell-cell adhesion during nephron development. Cell Reports, Vol. 36, Issue 1, 109340, JULY 06, 2021. DOI: https://doi.org/10.1016/j.celrep.2021.109340.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2021-07-19