Xenopus models for Human eye diseases
Molecular mechanism of CHRDL1-mediated X-linked megalocornea in humans and in Xenopus model.
Human Molecular Genet. June 1, 2015; 24 (11): 3119-32.
Thorsten Pfirrmann et al
The cornea on the front surface of the human eye is our “window to the world”, hence maintenance of corneal tissue transparency is essential for vision. The integrity and functionality of the outermost corneal layer, the epithelium, plays a key role in refraction of light onto the retina at the back of the eye.
Megalocornea (MGC1 or XMC) is an inherited eye disorder in which the corneal diameter is enlarged and anterior chamber of the eye is too deep. The condition is evident at birth, and mosaic corneal degeneration, cataracts and glaucoma develop in later life.
XMC is known to be caused by mutations in the Chordin-Like 1 (CHRDL1) gene, that encodes the BMP antagonist protein, Ventroptin, which is involved in cell fate determination and cell differentiation.
The goal of this study was to elucidate the molecular mechanism(s) involved in the development of the enlarged anterior part of the eye, by studying a large family with a range of XMC phenotypes, and to produce the first animal model for this disease using Xenopus laevis.
The authors first assessed the familiar pedigree, noting an X-linked recessive inheritance pattern accounted for the male preponderance of the disorder, and characterized the families XMC phenotypes. They identified the novel CHRDL1 frameshift mutation [c.807_808delTC] in the family in affected males, and in asymptomatic female carriers. This mutation was presumed to cause a loss of function.
In Xenopus tadpoles they demonstrated that chrdl1 is specifically expressed in the lens, plexiform layers of the optic cup and ciliary marginal zone stem cells of the eye (plus otic vesicle notochord and ventricular zone of the neural tube) at stage NF 37&38. The Xenopus chrdl1 knockdown phenocopied the human XMC phenotype. This confirmed that this CHRDL1 deficiency does cause XMC in people. Interestingly, they also showed that bmp4 is down regulated in the Xenopus eyes. Moreover, phospho-SMAD1/5 is altered and BMP receptor 1A is reduced in a XMC patient.
They authors thus propose a negative-feedback regulation due to the deficient BMP antagonism in XMC. As the epithelium of the cornea is maintained by stem cells, and CHRDL1 is preferentially expressed in these limbal stem cells of adult human cornea, the authors predict that CHRDL1 plays a key role in cornea homeostasis, and thus suggest that the limbal stem cell niche are promising targets for regenerative therapies of corneal injuries.
The Xenopus experiments in this study were performed by Thorsten Pfirrmann in the Holleman Lab, at the Martin Luther University Halle-Wittenberg, in Germany.
Disease(s): Megalocornea (MGC1 or XMC)
Genes associated with this disease:chrdl1
OMIM ID:#309300
Click here to see the Xenbase article page.
Click here to see the article on the HMG website.
____________________________
Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients.
Takuya Nakayama, Marilyn Fisher, Keisuke Nakajima, Akinleye Odeleye, Keith Zimmerman, Margaret Fish, Yoshio Yaoita, Jena Chojnowski, James Lauderdale, Peter Netland and Robert M Grainger.
Developmental Biology Available online 25 February 2015 In Press
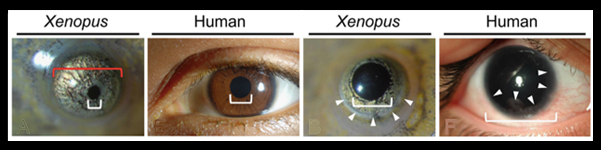
Aniridia is an inherited eye disorder characterized by a complete or partial absence of the iris, the colored part of the eye, and abnormal pupil shape, together causing reduced visual acuity and sensitivity to light. About 1 in 50-100,000 people suffer from aniridia. Patients frequently have additional symptoms( erratic eye moments, glaucoma, cataracts, optic nerve defects) that contribute to progressive vision loss. Aniridia is comorbid in a number of patients with tumors of the kidney, and in many cases, patients present with behavioral problems, developmental delays and problems detecting odors.
In this new paper by Takuya Nakayama et al. from the Grainger lab, TALEN constructs were used to target the pax6 gene in X. tropicalis. The authors demonstrate that severe eye phenotypes and changes in brain and pancreas development result. Homozygous pax6 mutants are embryonic lethal and have malformed retina and no lens. Partial loss of function froglets, those with hypomorphic pax6 mutations, have iris defects, cataracts and corneal defects that phenocopy human aniridia patients.
This research confirms the power of TALEN editing to rapidly manipulate the genome of Xenopus embryos. It also highlights the utility of Xenopus to understand the earliest effects on development of specific disease genes. In this study, gene expression of the Xenopus pax6 mutants demonstrate the role of pax6 in eye development and neural patterning, establishing another Xenopus human eye disease model.
Genes associated with this disease: pax6
OMIM ID:#106210 Aniridia (AN);#206700 Gillespie Syndrome, #194072 Wagr Syndrome (WAGR).
Click here to see the Xenbase article page.
Click here to see the article on the Developmental Biology website.
Last Updated: 2015-06-22