Phosphorylation Dynamics Dominate the Regulated Proteome during Early Xenopus Development
Sci Rep. November 15, 2017; 7 (1): 15647.
Peuchen EH, Cox OF, Sun L, Hebert AS, Coon JJ, Champion MM, Dovichi NJ, Huber PW.
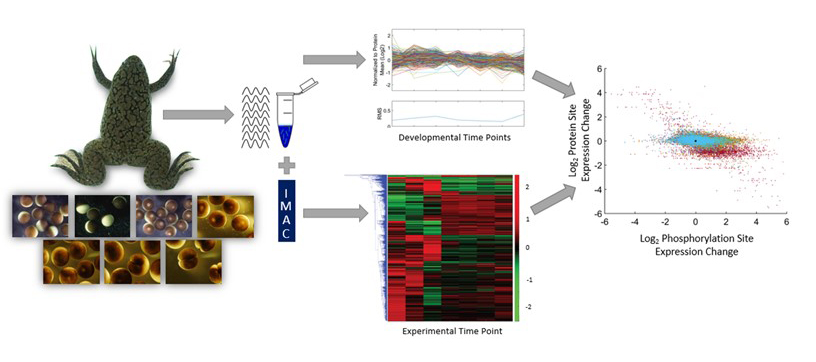
Quantitative measurements of phosphoproteome beginning with stage VI oocytes, oocyte maturation to a fertilizable egg, and three time points following fertilization.
Click here to view this article on Science Reports.
Click here to view this article on Pubmed.
Click here to view this article on Xenbase.
Abstract
The earliest stages of animal development are largely controlled by changes in protein phosphorylation mediated by signaling pathways and cyclin-dependent kinases. In order to decipher these complex networks and to discover new aspects of regulation by this post-translational modification, we undertook an analysis of the X. laevis phosphoproteome at seven developmental stages beginning with stage VI oocytes and ending with two-cell embryos. Concurrent measurement of the proteome and phosphoproteome enabled measurement of phosphosite occupancy as a function of developmental stage. We observed little change in protein expression levels during this period. We detected the expected phosphorylation of MAP kinases, translational regulatory proteins, and subunits of APC/C that validate the accuracy of our measurements. We find that more than half the identified proteins possess multiple sites of phosphorylation that are often clustered, where kinases work together in a hierarchical manner to create stretches of phosphorylated residues, which may be a means to amplify signals or stabilize a particular protein conformation. Conversely, other proteins have opposing sites of phosphorylation that seemingly reflect distinct changes in activity during this developmental timeline.
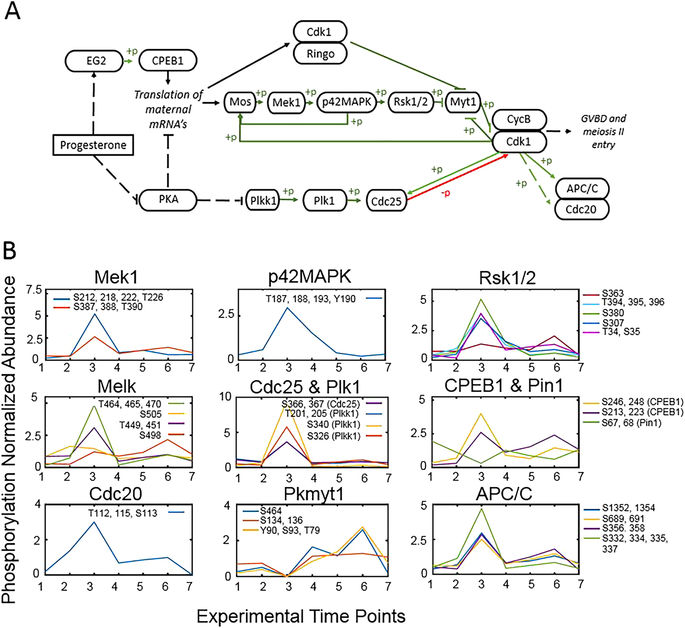
Figure 5. Phosphorylation events during progesterone-dependent oocyte maturation. (A) Outline of the major members of the signaling pathway for oocyte maturation initiated by progesterone adapted from87. Phosphorylation events are represented in green and dephosphorylation in red with arrows indicating activation and bars indicating repression. Pathways with intermediates not shown are indicated with a broken line. (B) The occupancy of individual phosphorylation sites in proteins within the pathway is presented relative to the seven experimental time points. Changes are normalized to the mean of the individual phosphopeptide. Inserts of each panel identify the detected sites of phosphorylation.
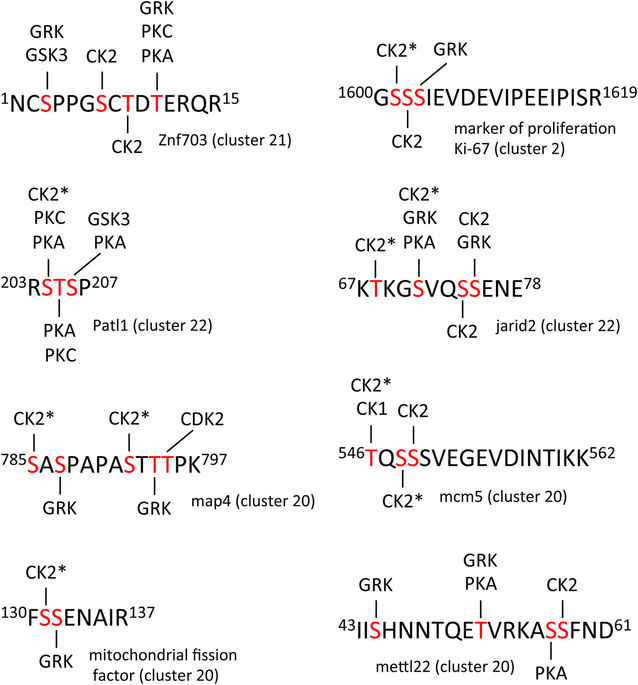
Figure 7. Clustered sites of phosphorylation. A selection of multiple-phosphorylated peptides is presented. Phosphorylation sites indicated in red had a minimum fractional occupancy of >0.1. Predicted kinase substrate sites were identified using PhosphoMotif Finder86. GRK (G protein-coupled receptor kinase), GSK3 (glycogen synthase kinase 3), CK2 (casein kinase 2), PKC (protein kinase C), PKA (protein kinase A), CDK2 (cyclin-dependent kinase 2), CK1 (casein kinase 1). CK2* indicates that phosphorylation at this site requires prior phosphorylation of a proximal priming site.
Adapted with permission from Scientific Reports: Peuchen et al. (2017). Phosphorylation Dynamics Dominate the Regulated Proteome during Early Xenopus Development. Sci Rep. November 15, 2017; 7 (1): 15647. Copyright 2017.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2017-12-13