Morpholino Side Effects
Dev Cell. February 17, 2018;
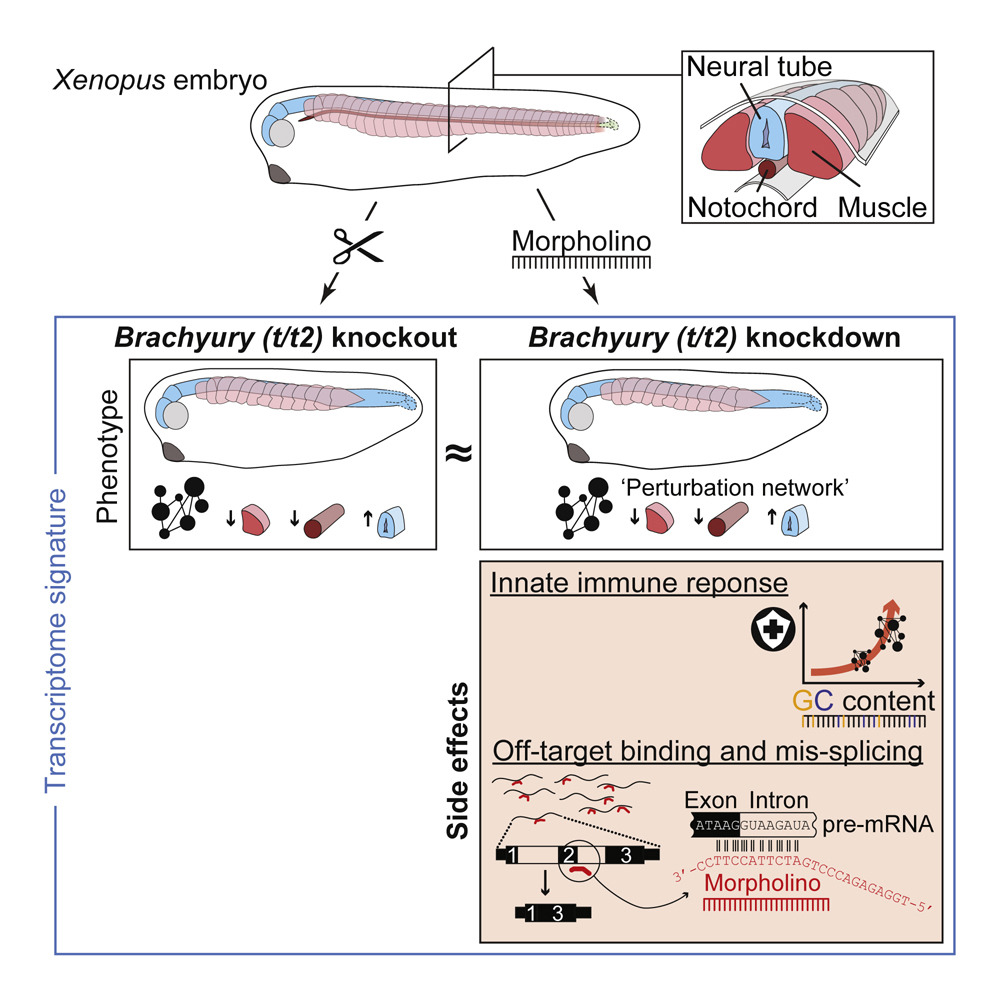
Innate Immune Response and Off-Target Mis-splicing Are Common Morpholino-Induced Side Effects in Xenopus
Gentsch GE, Spruce T, Monteiro RS, Owens NDL, Martin SR, Smith JC.
For almost two decades, developmental biologists have used antisense morpholino oligomers (MOs) to knock down gene function. Little is known, however, about the side effects of MOs. By systematically comparing the knockdown and knockout of Brachyury paralogues in X. tropicalis, Gentsch et al. show that embryos injected with control and target MOs show a GC content-dependent immune response and off-target splicing defects. These side effects are likely to be common, and at least some of them hard to eliminate.
Highlights
Brachyury KO and KD in frog equally affect phenotype-causing downstream genes.
Other transcriptional anomalies are unique to morpholino-based KDs and controls.
Morpholinos can trigger an innate immune response and off-target mis-splicing.
Optimization of KD conditions mitigates but does not eliminate these side effects.
* please see the follow-up article from Paraiso et al. (2019) as reported on Xenbase, Pubmed, and Developmental Cell
Click here to view article at Developmental Cell.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
Antisense morpholino oligomers (MOs) have been indispensable tools for developmental biologists to transiently knock down (KD) genes rather than to knock them out (KO). Here we report on the implications of genetic KO versus MO-mediated KD of the mesoderm-specifying Brachyury paralogs in the frog Xenopus tropicalis. While both KO and KD embryos fail to activate the same core gene regulatory network, resulting in virtually identical morphological defects, embryos injected with control or target MOs also show a systemic GC content-dependent immune response and many off-target splicing defects. Optimization of MO dosage and increasing incubation temperatures can mitigate, but not eliminate, these MO side effects, which are consistent with the high affinity measured between MO and off-target sequence in vitro. We conclude that while MOs can be useful to profile loss-of-function phenotypes at a molecular level, careful attention must be paid to their immunogenic and off-target side effects.
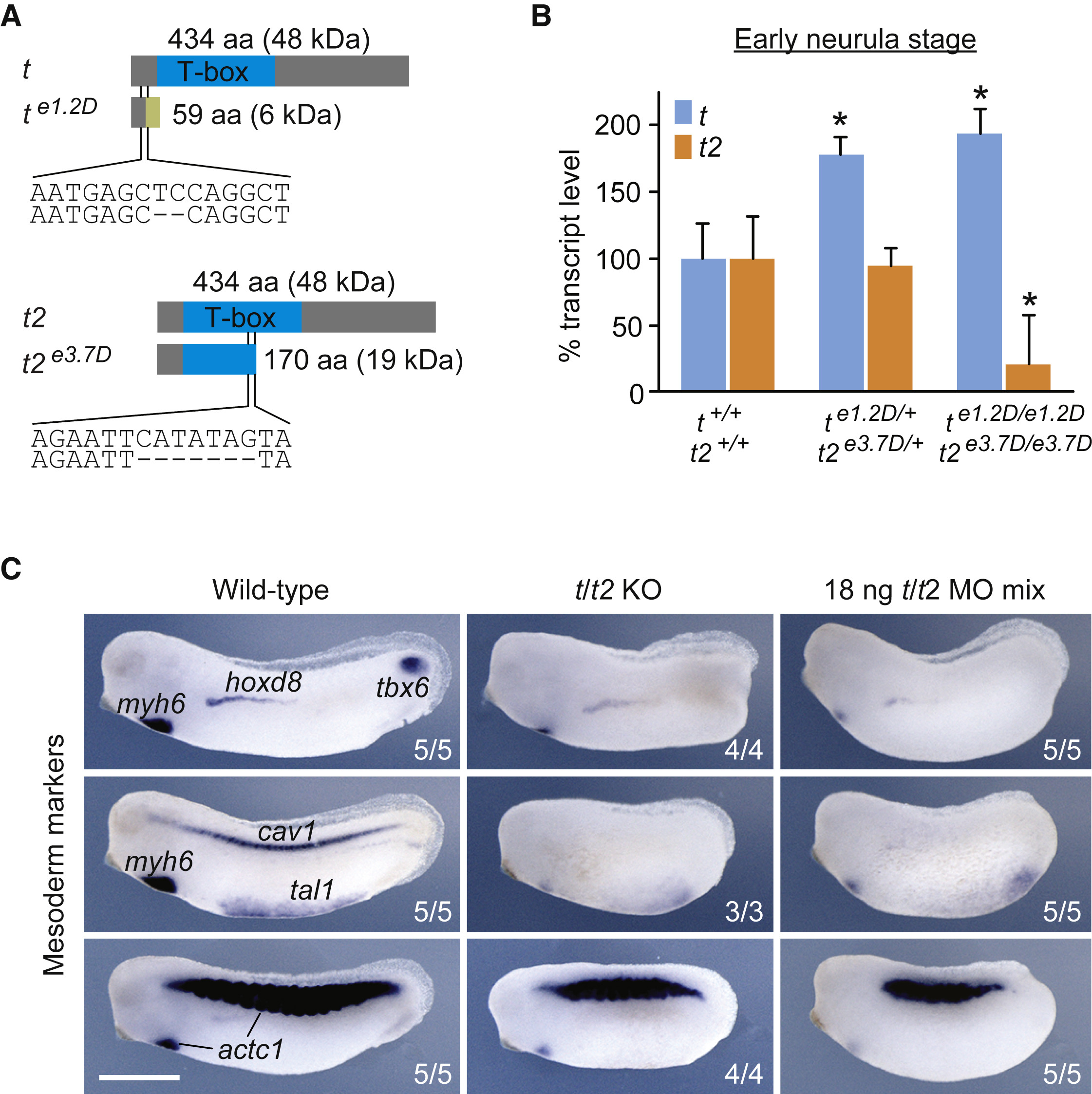
Figure 1. TALEN-Induced Deletions Nullify Brachyury Function (A) TALEN-induced 2- and 7-bp deletions in exon 1 of t (e1.2D) and exon 3 of t2 (e3.7D), and predicted frameshift translations generating truncated proteins of 59 and 170 amino acids (aa). These mutations were selected to generate a double heterozygous X. tropicalis line for the Brachyury paralogs t and t2 (te1.2D/+t2e3.7D/+). (B) t and t2 transcript levels in hetero- and homozygous embryos as measured by qRT-PCR at early neurula stage (n = 3, mean ± SD). Two-tailed t test: ∗p ≤ 0.05. (C) Multi-probe WMISH for various mesoderm cell lineage and derivative markers (actc1; cardiac and skeletal muscle; cav1, notochord; hoxd8, pronephros; myh6, heart; tal1, ventral blood island; tbx6, paraxial mesoderm) in wild-type and Brachyury (t/t2) null (KO) embryos, as well as embryos injected with four MOs targeting t and t2 (t/t2 MO mix) at mid-tailbud stage. Scale bar, 0.5 mm.
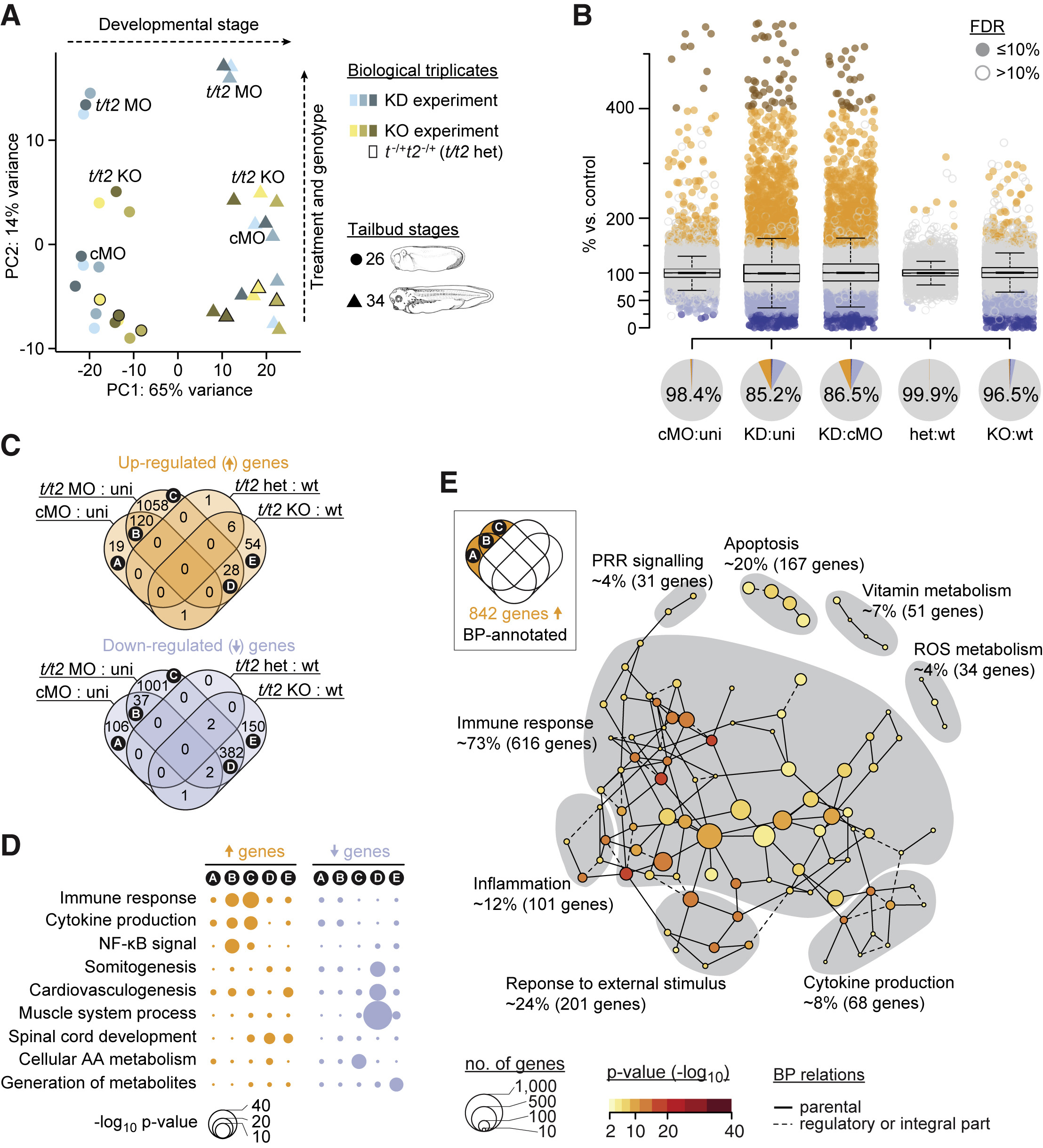
Figure 2. Transcriptional Deviation from Genetic Counterpart Reveals Immune Response as MO Side Effect (A) Principal component (PC) analysis of poly(A) RNA profiles at indicated tailbud stages resulting from biological triplicates of a t/t2 KD and KO experiment. The KD experiment involved uninjected (not labeled), control (cMO), and t/t2 MO-injected embryos. The KO experiment consisted of wild-type (WT) (not labeled), heterozygous (t−/+t2−/+; data points framed in black, not labeled) and homozygous (t/t2 KO) embryos. (B) Jitter/boxplot and pie chart show pairwise transcriptional comparisons of cMO, t/t2 MO (KD), heterozygous (het), KO embryos with uninjected (uni), cMO, or wild-type (WT) embryos. Only fold changes with FDR ≤10% were colored: navy blue <25%, sky blue 25%–67%, orange 150%–400%, and red >400% compared with reference transcript level. Percentage number in pie chart represents percentage of genes that were unaffected (i.e., whose fold change are <1.5 or FDR >10% between indicated conditions). (C) Venn diagram of genes with increased and decreased transcript levels (i.e., ≥1.5-fold change at FDR ≤10%). See Table S3 for corresponding gene list. (D) Statistical significance (hypergeometric p value) of enrichment for some selected biological processes (BPs) among the indicated Venn fields. (E) MO-triggered transcriptional signature of an immune response. Gray areas represent Newman-Girvan-based communities of enriched BPs associated with 842 genes in fields A, B, and C of the Venn diagram. See Table S4 for corresponding and other Venn field-specific gene set enrichment analyses.
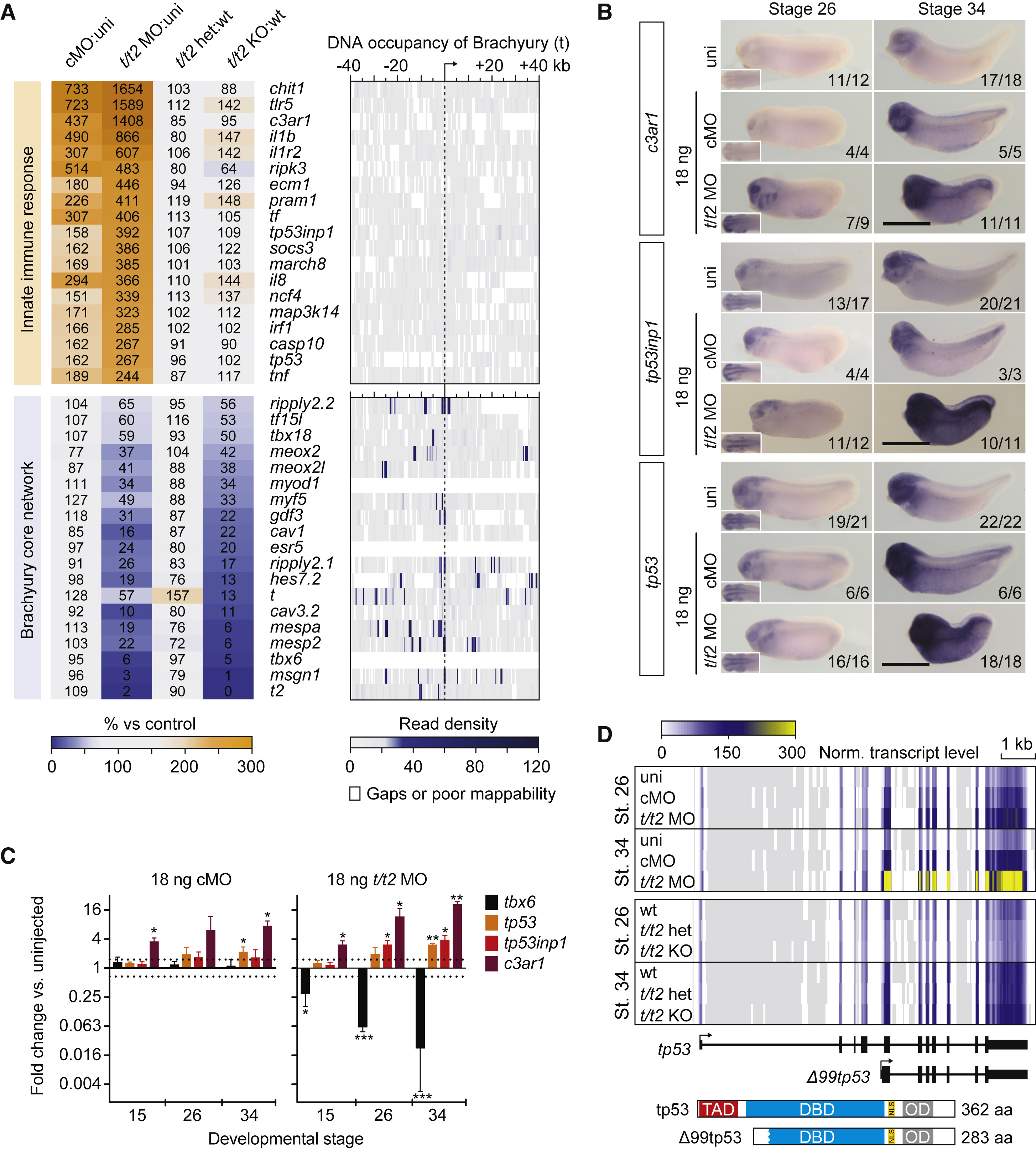
Figure 3. Ubiquitous Immune Response against MO Intensifies during Embryogenesis (A) Panel of genes upregulated in control and t/t2 morphants associated with the immune response and genes downregulated in t/t2 morphants and null mutants representing the Brachyury-dependent core network. Heatmap to the right represents the binding map of Brachyury (t) in the proximity (±40 kb) of indicated transcription start sites (TSS) at early tailbud stage (Gentsch et al., 2013). (B) WMISH of immune response related gene transcripts c3ar1, tp53inp1, and tp53 in uninjected (uni) embryos and embryos injected with 18 ng of cMO or t/t2 MO mix. Left bottom corner inset, dorsal view of tailbud head showing elevated transcript levels in the CNS. tp53 antisense probe did not discriminate active isoforms shown in D. Scale bar, 0.5 mm. (C) Temporal dynamics of transcript fold changes (log2 scale) for a selected group of genes representing the Brachyury-directed core network (tbx6) and the immune response (c3ar1, tp53inp1, and tp53) in MO-injected versus uninjected embryos as measured by qRT-PCR (n = 3, mean ± SD). Two-tailed t test (≥1.5-fold change): ∗p ≤ 0.1; ∗∗p ≤ 0.01; and ∗∗∗p ≤ 0.001. (D) Genome map of full length tp53 and Δ99tp53 transcript isoforms shows normalized transcript levels for uninjected (uni), control morphants (cMO), t/t2 morphants (t/t2 MO), wild-type (WT), t/t2 heterozygous (t/t2 het), and homozygous (t/t2 KO) mutant embryos at tailbud stages 26 and 34. Isoform-corresponding translation products with critical domains are on display below the heatmap: TAD, transactivation domain; DBD, DNA binding domain; NLS, nuclear localization signal; and OD, oligomerization domain.
Adapted with permission from Elsevier: Gentsch et al. (2018). Innate Immune Response and Off-Target Mis-splicing Are Common Morpholino-Induced Side Effects in Xenopus. Developmental Cell, Volume 44, Issue 5, 12 March 2018, Pages 597-610.e10, copyright (2018).
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
