In vivo microtomography of gastrulation
X-ray phase-contrast in vivo microtomography probes new aspects of Xenopus gastrulation
Julian Moosmann, Alexey Ershov, Venera Altapova, Tilo Baumbach, Maneeshi S. Prasad, Carole LaBonne, Xianghui Xiao, Jubin Kashef & Ralf Hofmann
Nature volume 497, pages 374–377 (16 May 2013).
The study of gastrulation in Xenopus, the stage at which the embryo has formed three layers arranged around a central cavity, has been hampered by the lack of high-quality live-imaging methods useable on intact Xenopus embryos, which are opaque at early stages.
Julian Moosmann, Ralf Hofmann, Jubin Kashef & their colleagues (Karlsruhe Institute of Technology), Xianghui Xiao (Argonne National Laboratory, IL), Alexey Ershov (National Research Tomsk Polytechnic University) and Maneeshi Prasad & Carole LaBonne (Northwestern University, IL) developed a non-invasive in vivo time-lapse phase-contrast X-ray microtomography technique which allows the observation of gastrulation. By analysing individual cell trajectories, collective tissue motion and the evolution of morphological features, the authors visualize known gastrulation movements and reveal the formation of a transient ectodermal ridge structure not reported on previously.
Text adapted from Nature Editor’s summary.
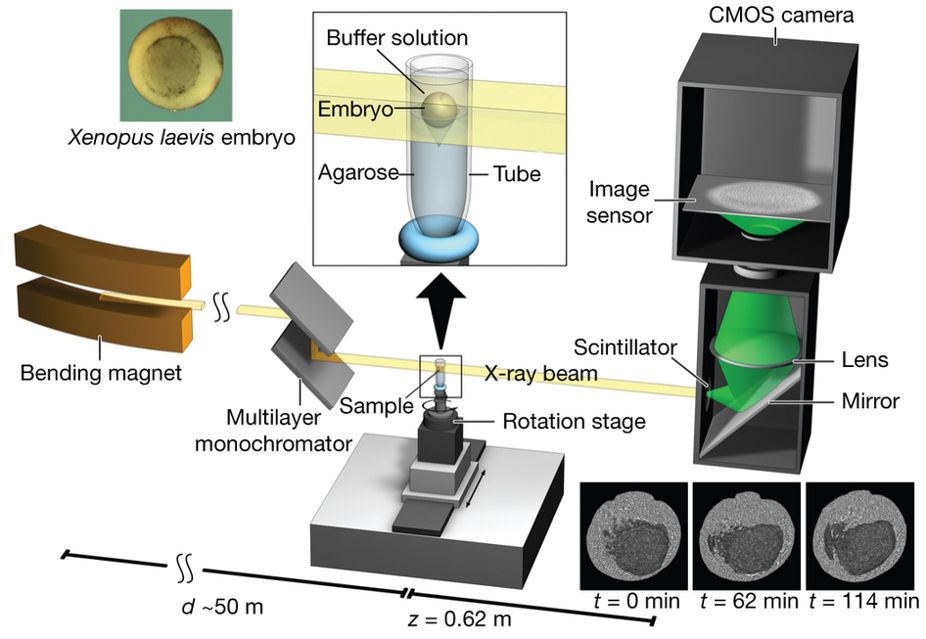
Figure 1 : Experimental set-up for propagation-based phase-contrast X-ray microtomography.
A quasi-parallel photon beam is generated from a synchrotron electron beam traversing the field of a bending magnet. After beam shaping and monochromatization, X-ray wave fronts propagate over a distance d (∼50 m) to impinge on the sample (living X. laevis embryo immersed in buffer solution and suspended by agarose) mounted on a rotation stage for tomographic data acquisition. The 2D detector, at a distance z = 62 cm behind the sample, consists of a scintillator, converting X-rays into visible light, followed by a mirror, a lens, and a complementary metal oxide semiconductor (CMOS) camera (effective pixel size Δx = 2.2 µm).
Click here to view article at Nature.
Click here to view article on Xenbase.
Time-lapse volumes (4D) of the processed data can be downloaded from Xenbase : ftp://xenbaseturbofrog.org/videos/moosmann_et_al_2013/
Click on image to play video on browser from the Nature website, or right-click and save link as mp4 video to play on your computer.
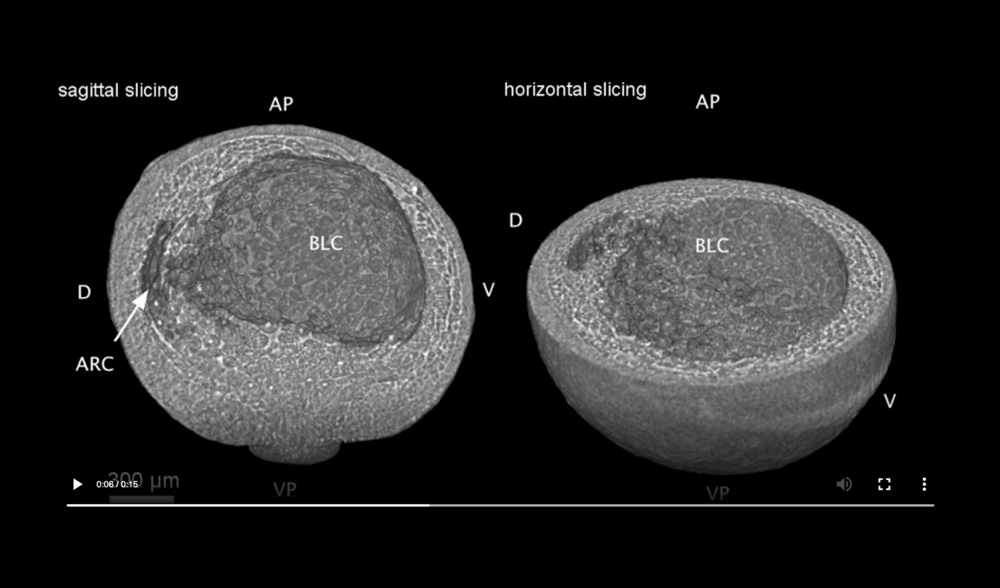
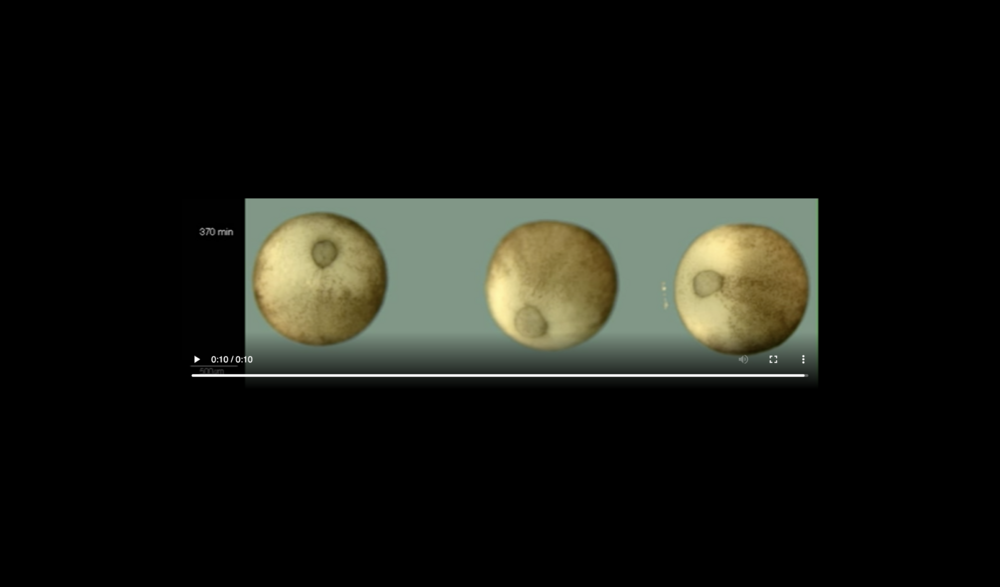
Abbreviations: archenteron (ARC), dorsal and ventral sides (D,V), blastocoel (BLC), animal pole (AP), and vegetal pole (VP). Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
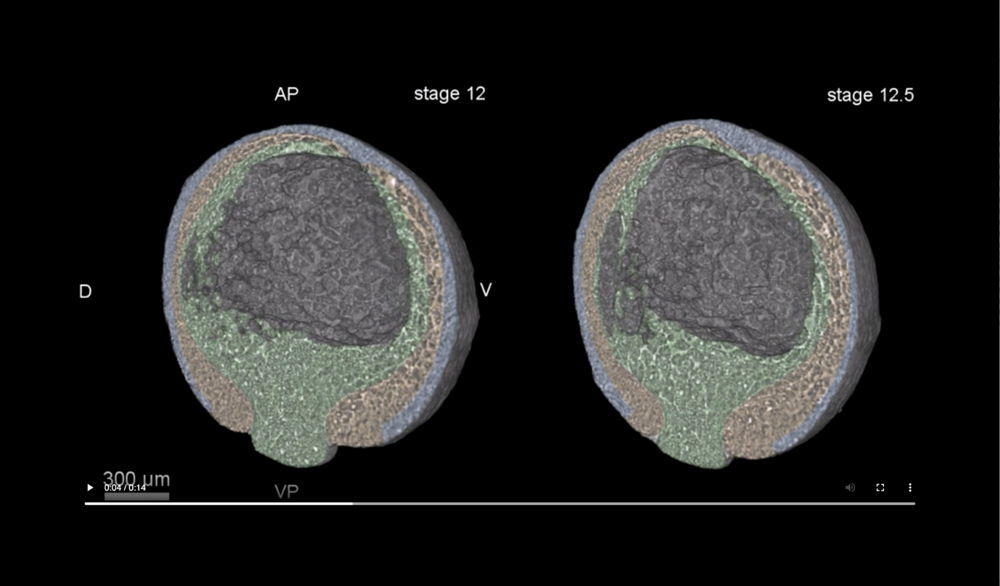
Ectoderm (blue), mesoderm (orange), and endoderm (green). Abbreviations: dorsal and ventral sides (D,V), animal pole (AP), and vegetal pole (VP). Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
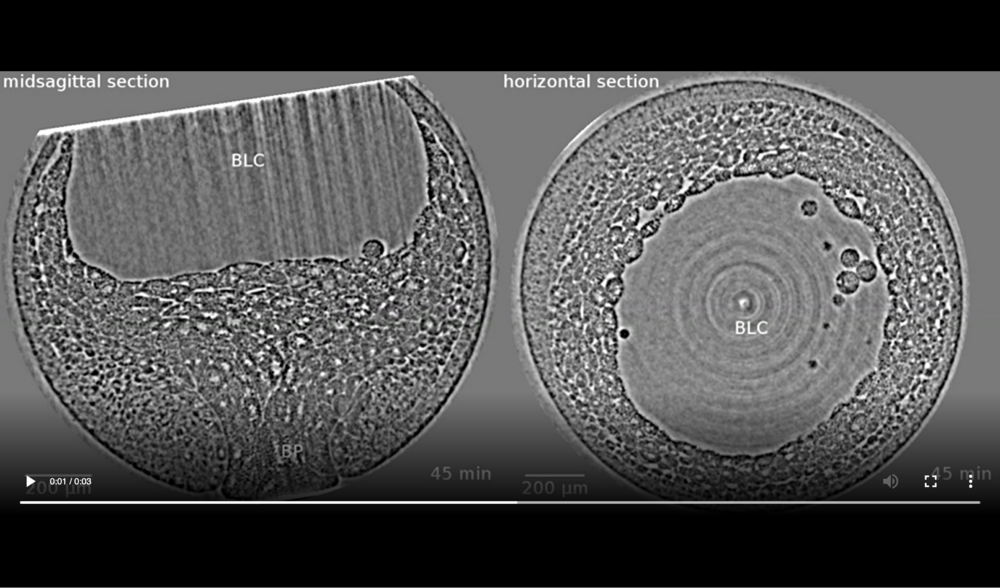
Following structures are labelled: blastopore (BP), blastocoel (BLC), and archenteron (ARC). Jumps are related to fact that four (times 21, 42, 93, and 124 min) out of thirteen volumes were corrupted (software flaw in rotation-stage control during exposure) and thus had to be dismissed, resulting in a time-lapse series with variable time steps (10 or 20 minutes). Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
Right panel shows slices one cell layer away from blastocoel floor, crawling of cells on blastocoel floor can be seen. Following structures are labelled: blastopore (BP) and blastocoel (BLC). Data taken at beamline 32-ID of Advanced Photon Source (E=34 keV, z=70 cm, N=834, ΔE/E=10-4, 20 ms exposure time per projection, and flux density ~2˙1012 photons/mm2/s). Archenteron did not yet inflate (natural variations in archenteron morphogenesis, see [4]), it is shorter than the one shown in Supplementary Video 3 due to a higher flux density, a shorter waiting time, and a larger exposure time per projection.
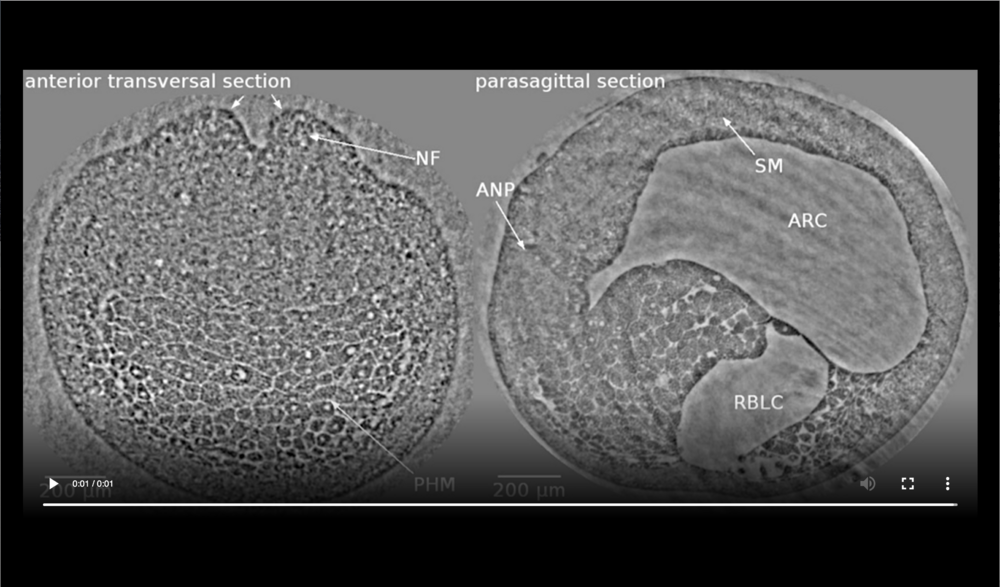
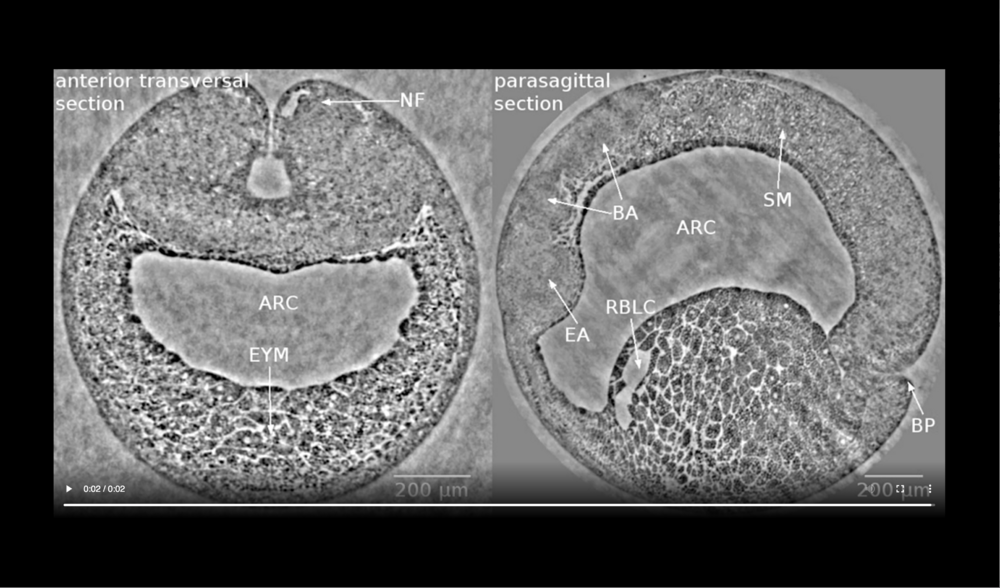
Left panel: dorsal at top, neural tube closure can be observed, white arrows define edges of neural folds (NF), prospective head endoderm (PHM) remains static; Right panel: dorsal at top, anterior to left, following tissues and cavities can be distinguished: anterior part of neural plate (ANP), somitogenic mesoderm (SM), remnant of blastocoel (RBLC), and archenteron (ARC). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s). Two tomograms were dismissed since embryo slid down the buffer cone (0 min) and due to software flaw during data acquisition (21 min).
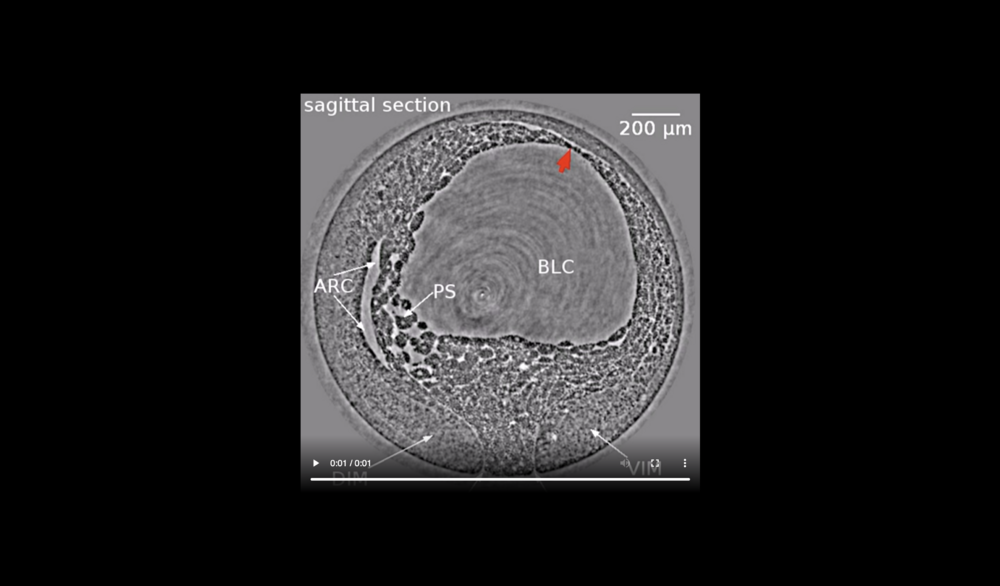
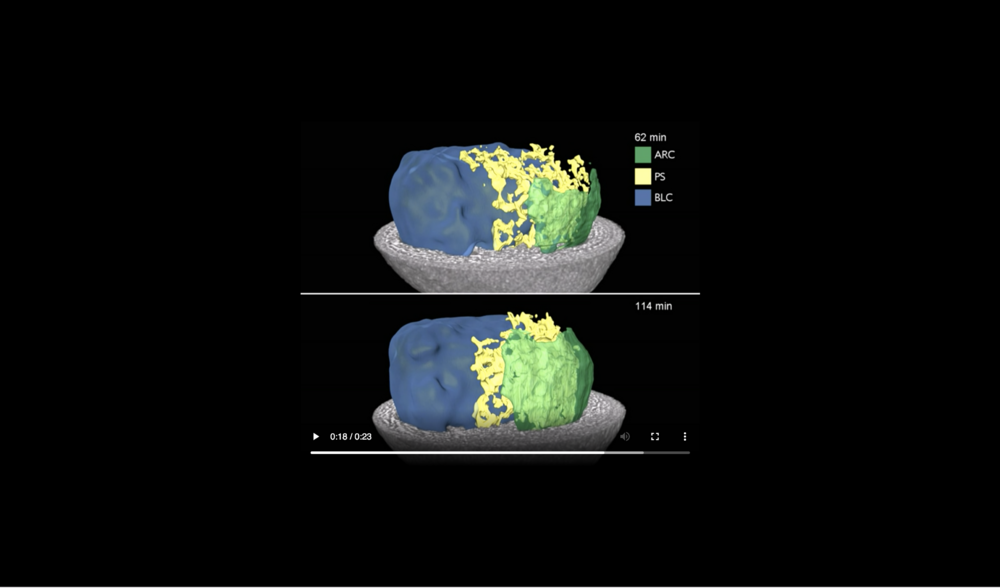
Animal at top, dorsal to left, following tissues and cavities can be distinguished: dorsal involuting mesendoderm (DIM), ventral involuting mesendoderm (VIM), blastocoel (BLC), “pipe” system of porous, endodermal tissue in between archenteron and blastocoel (PS), and archenteron (ARC), closure of the blastopore (white arrows), confrontation zone between head and ventral mesendoderm (red arrowhead). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s).
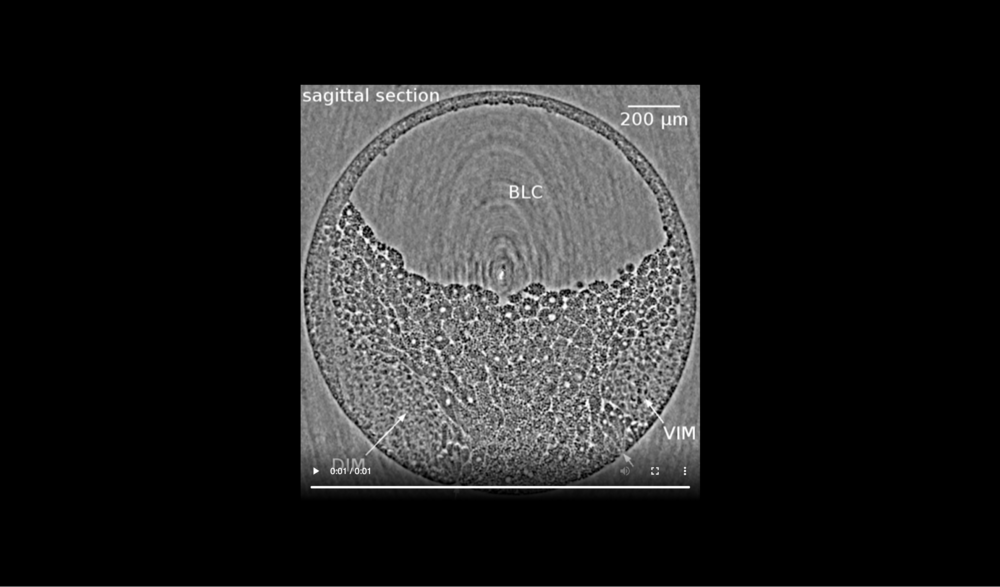
Animal at top, dorsal to left, following tissues and cavities can be distinguished: dorsal involuting mesendoderm (DIM), ventral involuting mesendoderm (VIM), blastocoel (BLC), closure of the blastopore (white arrows). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s). Two tomograms were dismissed due to software flaws during data acquisition (12 and 68 min).
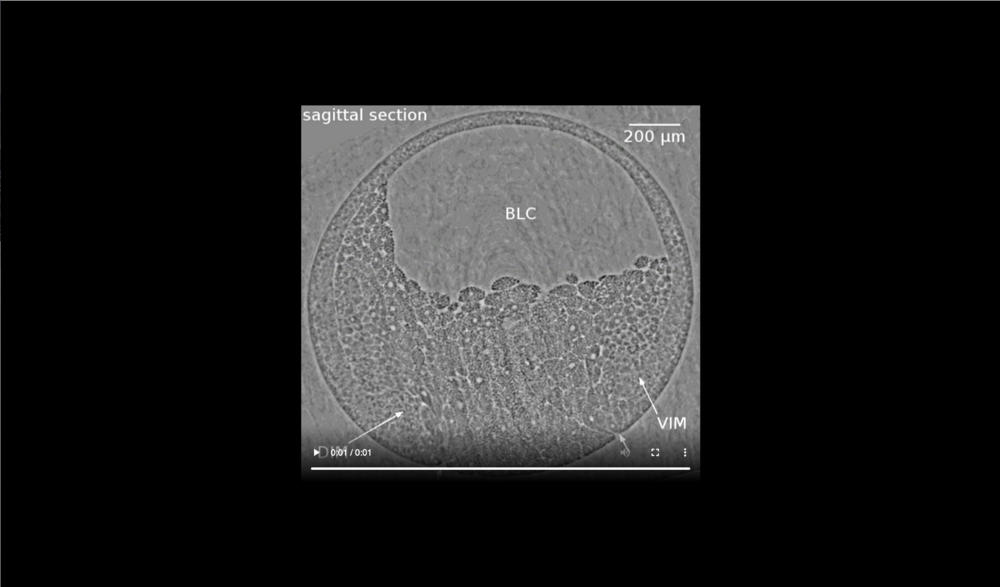
Animal at top, dorsal to left, following tissues and cavities can be distinguished: dorsal involuting mesendoderm (DIM), ventral involuting mesendoderm (VIM), blastocoel (BLC), closure of blastopore (white arrows). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s).
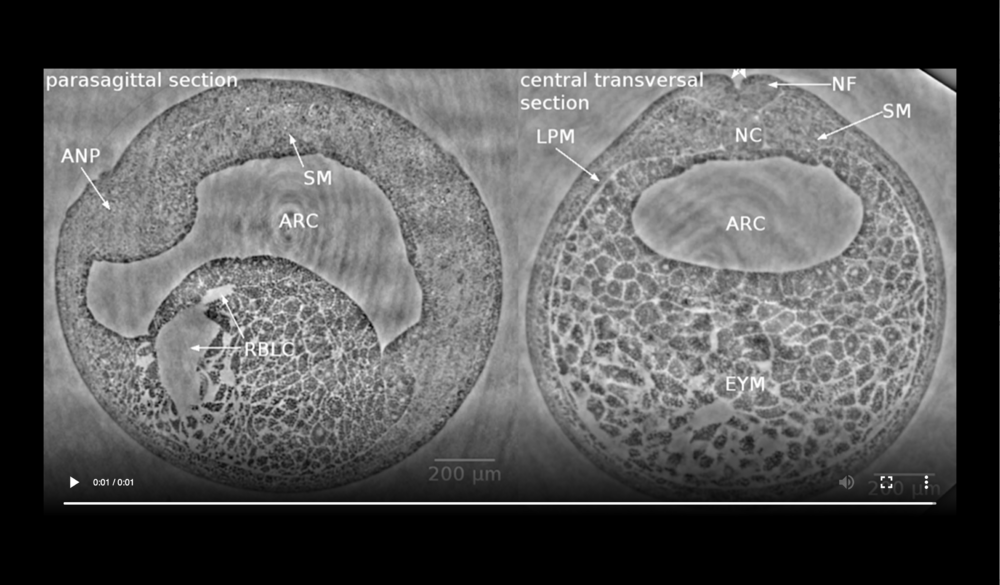
Left panel: dorsal at top, anterior to left, following tissues and cavities can be distinguished: anterior part of neural plate (ANP), somitogenic mesoderm (SM), remnants of blastocoel (RBLC), and archenteron (ARC); Right panel: dorsal at top, following tissues and cavities can be distinguished: notochord (NC), neural fold (NF), somitogenic mesoderm (SM), lateral plate mesoderm (LPM), archenteron (ARC), and endodermal yolk mass (EYM). Neural tube closure can be observed (white arrows). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s).
Left panel: dorsal at top, following tissues and cavities can be distinguished: neural fold (NF), archenteron (ARC), and endodermal yolk mass (EYM); Right panel: dorsal at top, anterior to left, following tissues and cavities can be distinguished: eye anlage (EA), brain anlage (BA), somitogenic mesoderm (SM), remnant of blastocoel (RBLC), archenteron (ARC), and blastopore (BP). Data taken at 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, and flux density 1012 photons/mm2/s).
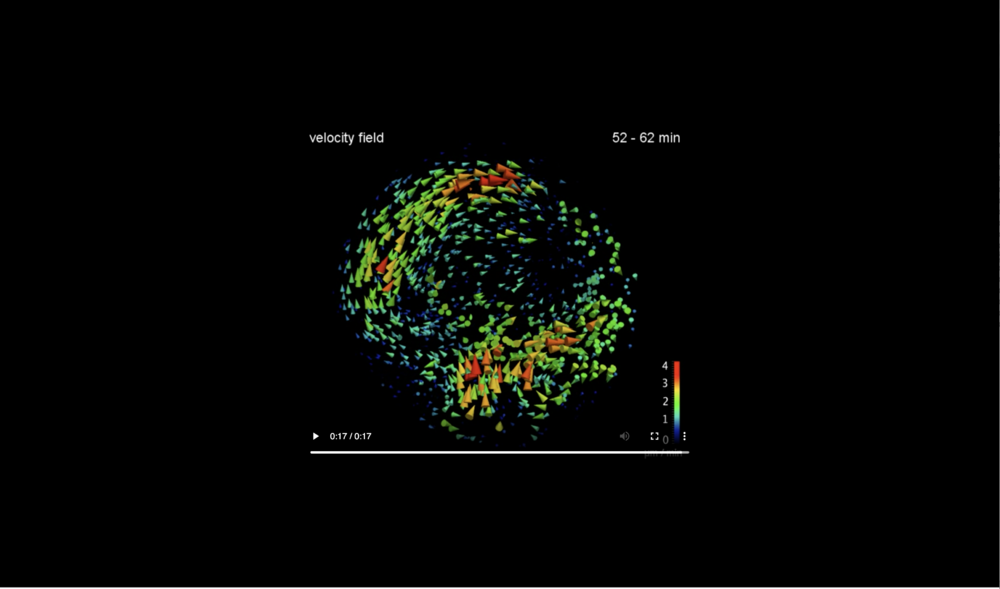
For better visibility the flow field is shown on the midsagittally halved embryo volume only. Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
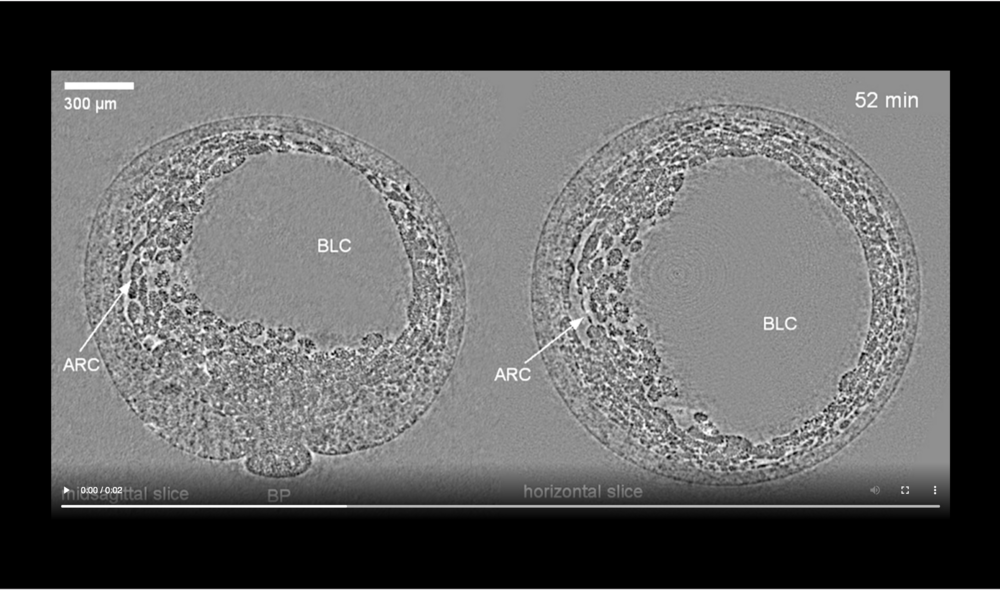
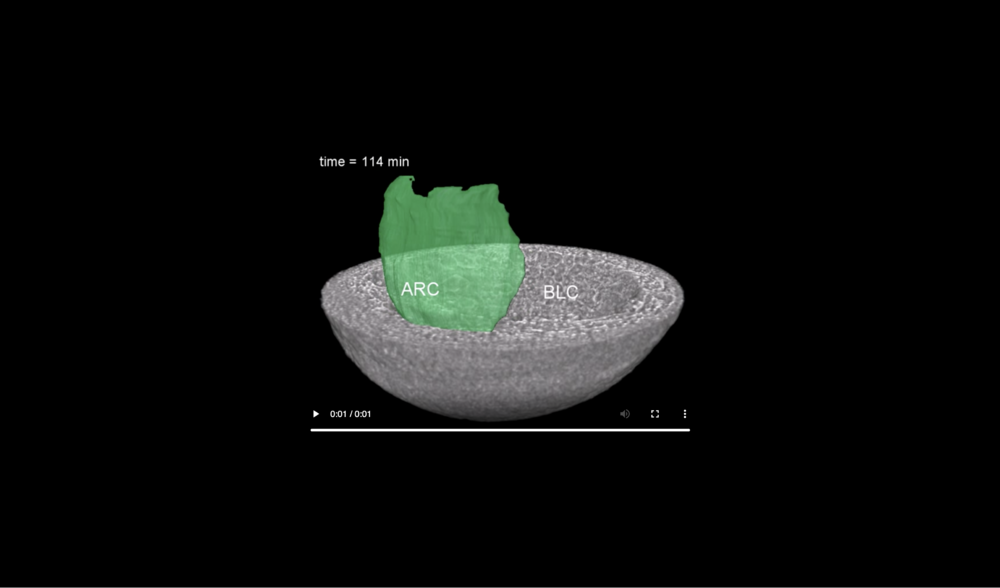
Abbreviations: archenteron (ARC) and blastocoel (BLC). Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
Embryos prefer to turn to their vegetal sides necessitating a manual swap to re-orientate them properly during the time-lapse measurements. This leads to slightly different viewing angles of the blastopores.
Abbreviations: archenteron (ARC), blastocoel (BLC), and “pipe” system (PS) of porous endoderm in between ARC and BLC. Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
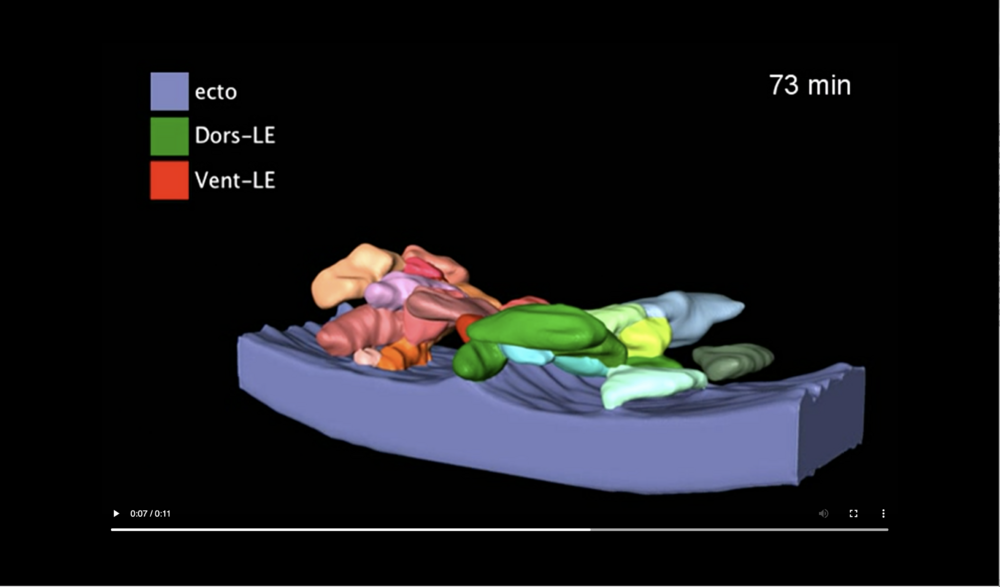
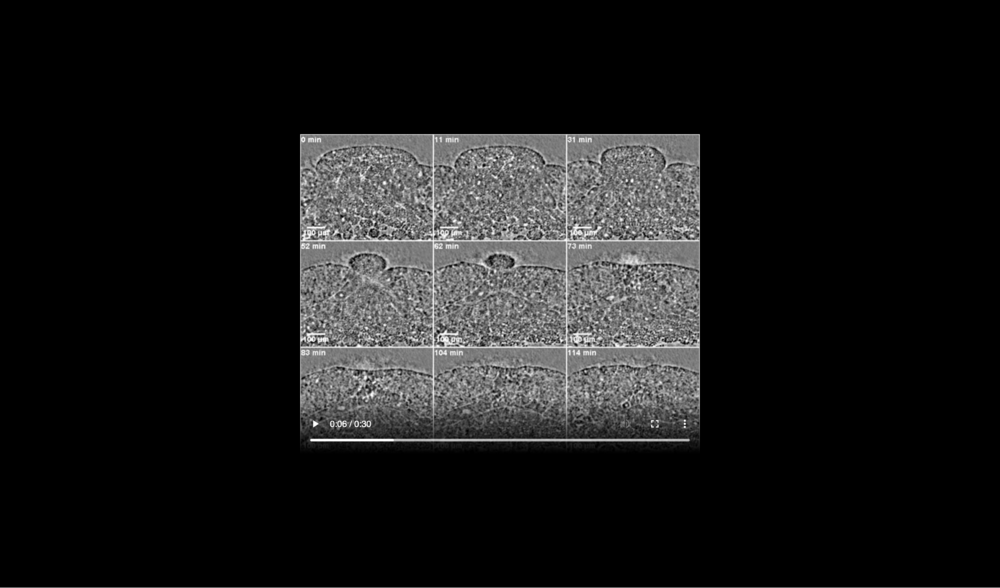
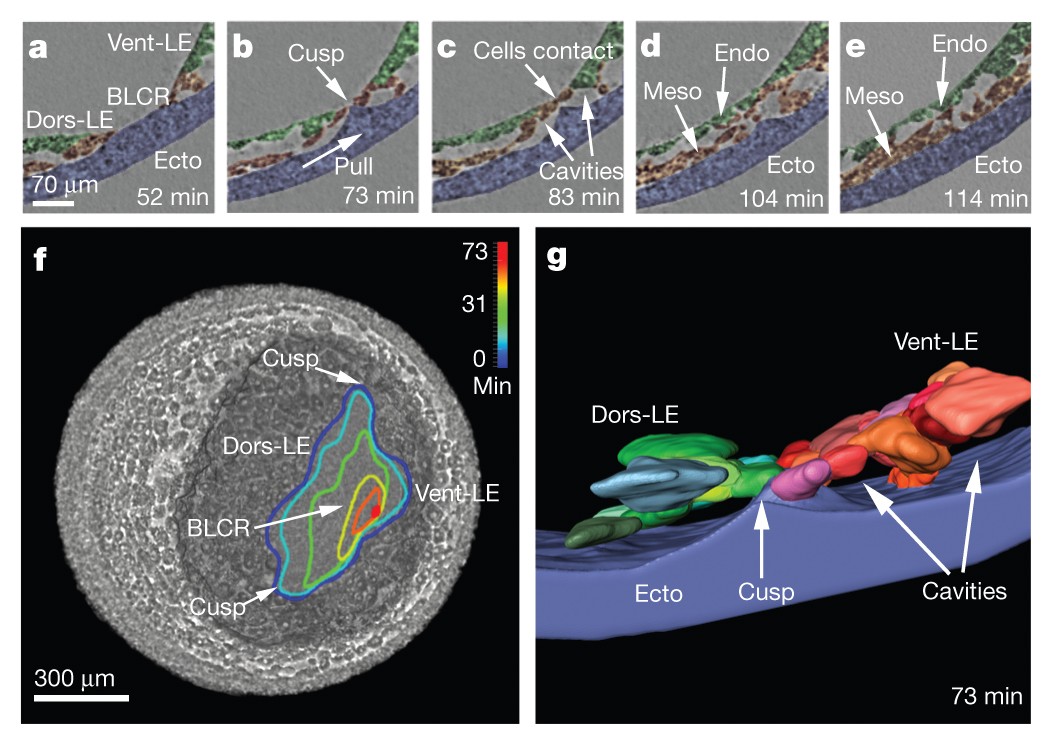
The transient ridge of ectoderm is fully pronounced at this time. Abbreviations: dorsal and ventral leading edges (Dors-LE, Vent-LE). Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
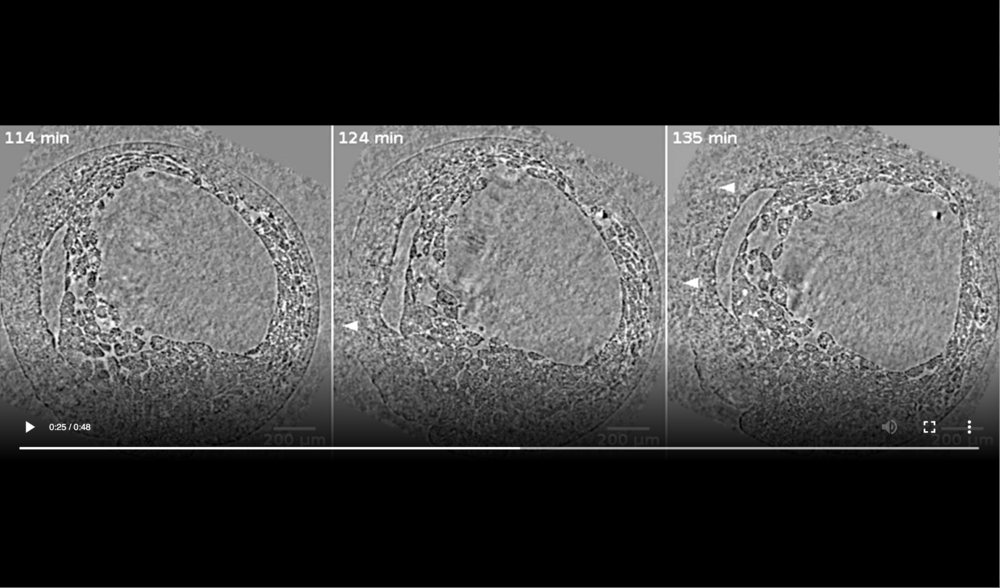
Left panel: embryo is intact at 114 min; Middle panel: cells ooze out of raptures in dorsal ectoderm (white arrowheads) at 124 min; Right panel: decay of embryo has progressed on the dorsal side giving rise to massive cell outflow (white arrowheads) at 135 min. Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
Small cavities disappear during blastopore closure suggesting that blastopore becomes watertight before onset of archenteron inflation. Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
Formation of ectodermal cusp and overlap of head and ventral mesendoderm are visible at late times. Data taken at station 2-BM-B of Advanced Photon Source (E=30 keV, z=62 cm, N=1200, ΔE/E=10-2, 15 ms exposure time per projection, and flux density 1012 photons/mm2/s).
Adapted with permission from Macmillan Publishers Ltd: Moosmann et al. (2013). X-ray phase-contrast in vivo microtomography probes new aspects of Xenopus gastrulation. Screen captures of the videos are from the supplementary information. Nature: 497,374–377 (16 May 2013) doi:10.1038/nature12116 , copyright (2013).
Last Updated: 2019-09-01