Ttc30a affects tubulin modifications in a model for ciliary chondrodysplasia with polycystic kidney disease
Getwan M, Hoppmann A, Schlosser P, Grand K, Song W, Diehl R, Schroda S, Heeg F, Deutsch K, Hildebrandt F, Lausch E, Köttgen A, Lienkamp SS.
Proc Natl Acad Sci USA. 2021 Sep 28;118(39):e2106770118. doi: 10.1073/pnas.2106770118.
Click here to view article at PNAS.
Click here to view article on Pubmed.
Click here to view article on Xenbase.

Abstract
Skeletal ciliopathies (e.g., Jeune syndrome, short rib polydactyly syndrome, and Sensenbrenner syndrome) are frequently associated with nephronophthisis-like cystic kidney disease and other organ manifestations. Despite recent progress in genetic mapping of causative loci, a common molecular mechanism of cartilage defects and cystic kidneys has remained elusive. Targeting two ciliary chondrodysplasia loci (ift80 and ift172) by CRISPR/Cas9 mutagenesis, we established models for skeletal ciliopathies in Xenopus tropicalis Froglets exhibited severe limb deformities, polydactyly, and cystic kidneys, closely matching the phenotype of affected patients. A data mining-based in silico screen found ttc30a to be related to known skeletal ciliopathy genes. CRISPR/Cas9 targeting replicated limb malformations and renal cysts identical to the models of established disease genes. Loss of Ttc30a impaired embryonic renal excretion and ciliogenesis because of altered posttranslational tubulin acetylation, glycylation, and defective axoneme compartmentalization. Ttc30a/b transcripts are enriched in chondrocytes and osteocytes of single-cell RNA-sequenced embryonic mouse limbs. We identify TTC30A/B as an essential node in the network of ciliary chondrodysplasia and nephronophthisis-like disease proteins and suggest that tubulin modifications and cilia segmentation contribute to skeletal and renal ciliopathy manifestations of ciliopathies in a cell type-specific manner. These findings have implications for potential therapeutic strategies.
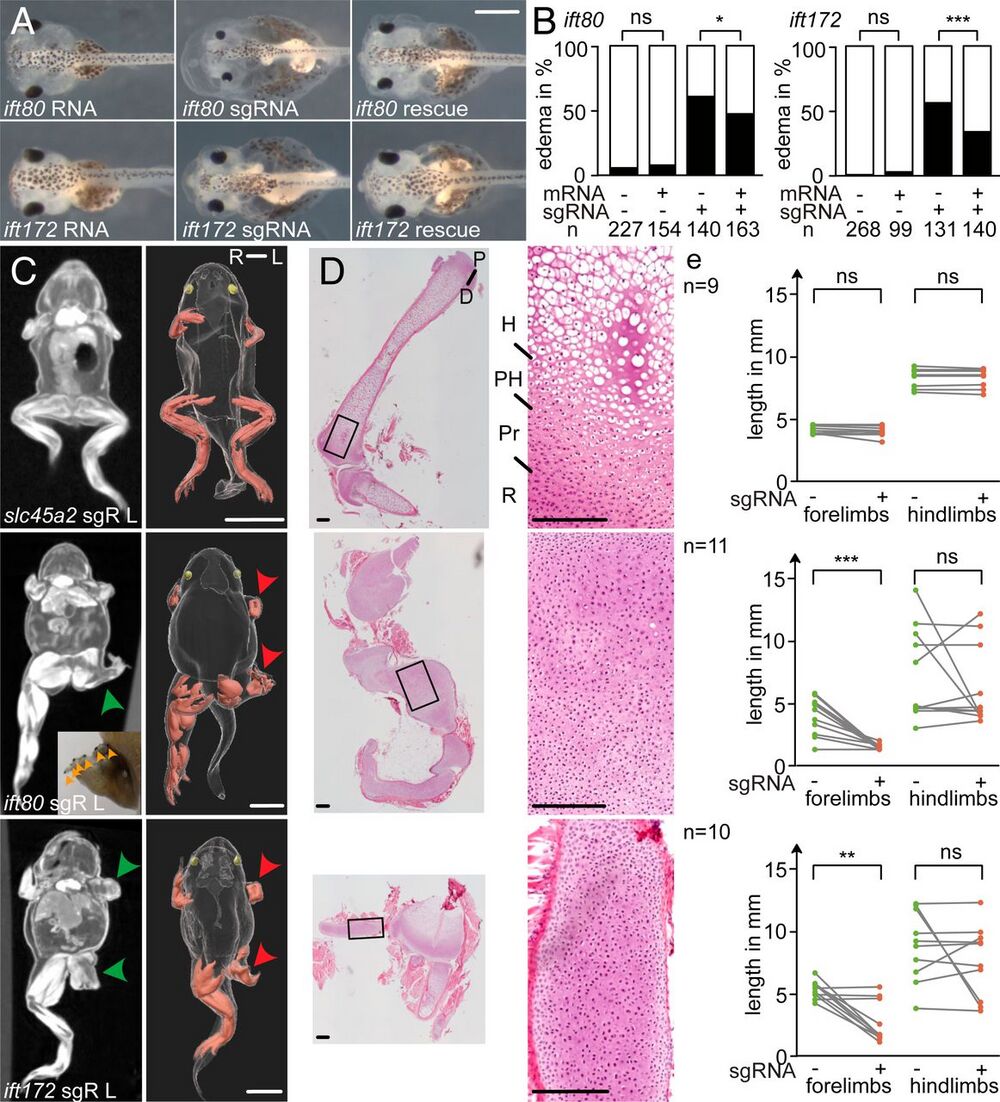
Fig. 1. CRISPR targeting of ift80 and ift172 leads to skeletal defects in X. tropicalis. (A and B) Analysis of edema formation in ift80 and ift172 crispants at stage 42. Note the smaller eyes in ift80-targeted embryos. (C) Limb phenotypes in postmetamorphic froglets (stage 62/63). MicroCT scans of representative crispant froglets of slc45a2 (negative control), ift80, and ift172 revealed cartilage accumulations in CRISPR-targeted froglets (green arrowheads). Injections of RNPs were performed unilaterally (Left) at the 2-cell stage. Polydactyly is indicated by orange arrowheads that mark black nailed digits. In the corresponding 3-dimensional reconstructions, limb muscles are shown in red, eyes in yellow, and red arrowheads point to the affected limbs. sgR L: left-sided sgRNA injection, R: right, L: left. (D) Histological sections of hindlimbs were stained with hematoxylin-eosin. R: resting zone; Pr: proliferating zone; PH: prehypertrophic zone; and H: hypertrophic zone. (E) The length of uninjected (green dots) versus injected (red dots) limbs of the same individual are plotted (gray lines). ns: (not significant); *P P P A) 0.5 mm, (C) 5 mm, and (D) 200 µm.]
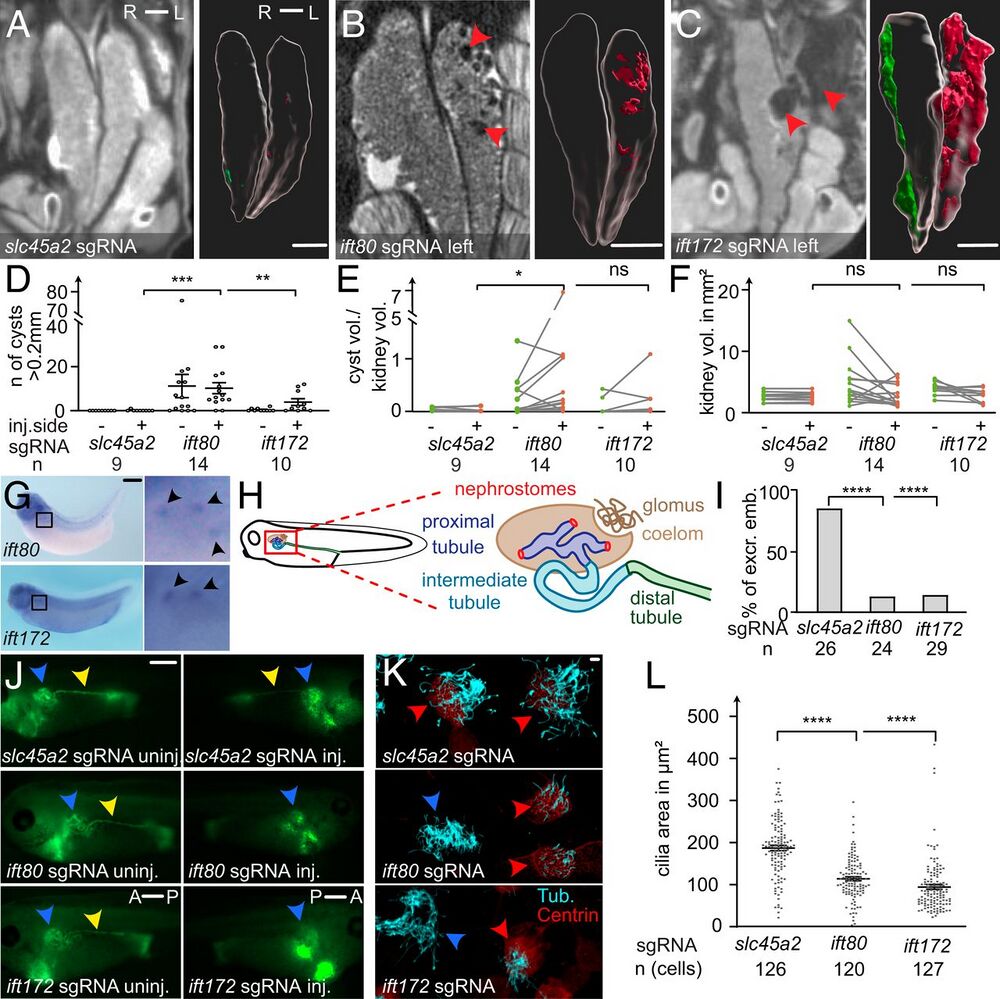
Fig. 2. Cystic kidney disease and cilia defects in ift80 and ift172 CRISPR–targeted X. tropicalis. (A–C) CRISPR/Cas9 targeting of slc45a2, ift80, and ift172 were performed unilaterally in two-cell–stage embryos. Mesonephroi of stage 61 to 63 froglets were analyzed by microCT scans and kidneys and cysts (red arrowheads in B and C) were segmented for 3D volumetric analysis; red: cysts on the injected side; green: uninjected side). (D) The number of cysts (>0.2mm), (E) the ratio of total cyst volume to kidney volume, and (F) the kidney volume (excluding cysts) was calculated and compared with kidneys of control injected animals. (G) Whole-mount in situ hybridization detects ift80 and ift172 in the multiciliated nephrostomes of the pronephros in stage 36 to 38 X. laevis. (H) Schematic depiction of the embryonic renal system of Xenopus. (I and J) Excretion assay with fluorescein-dextran at stage 38 to 40. Blue arrowheads point to the proximal part of the pronephros. Yellow arrowheads indicate fluorescence signal in the distal tubule, lacking on the injected side (J). A: anterior, P: posterior; excr: excreting; and emb: embryos. (K) Confocal images of multiciliated epidermal cells (MCCs) stained against acetylated tubulin (cyan). Centrin-RFP fusion protein served as a lineage marker (red arrowheads) and indicates CRISPR-targeted MCCs. Blue arrowheads point to nontargeted (wild type) cells. (L) The ciliated area was determined for each cell. Error bars indicate SEM. P > 0.05 ns (not significant); *P P P A–C) 1 mm, (G and I) 0.5 mm, and (L) 10 µm.]
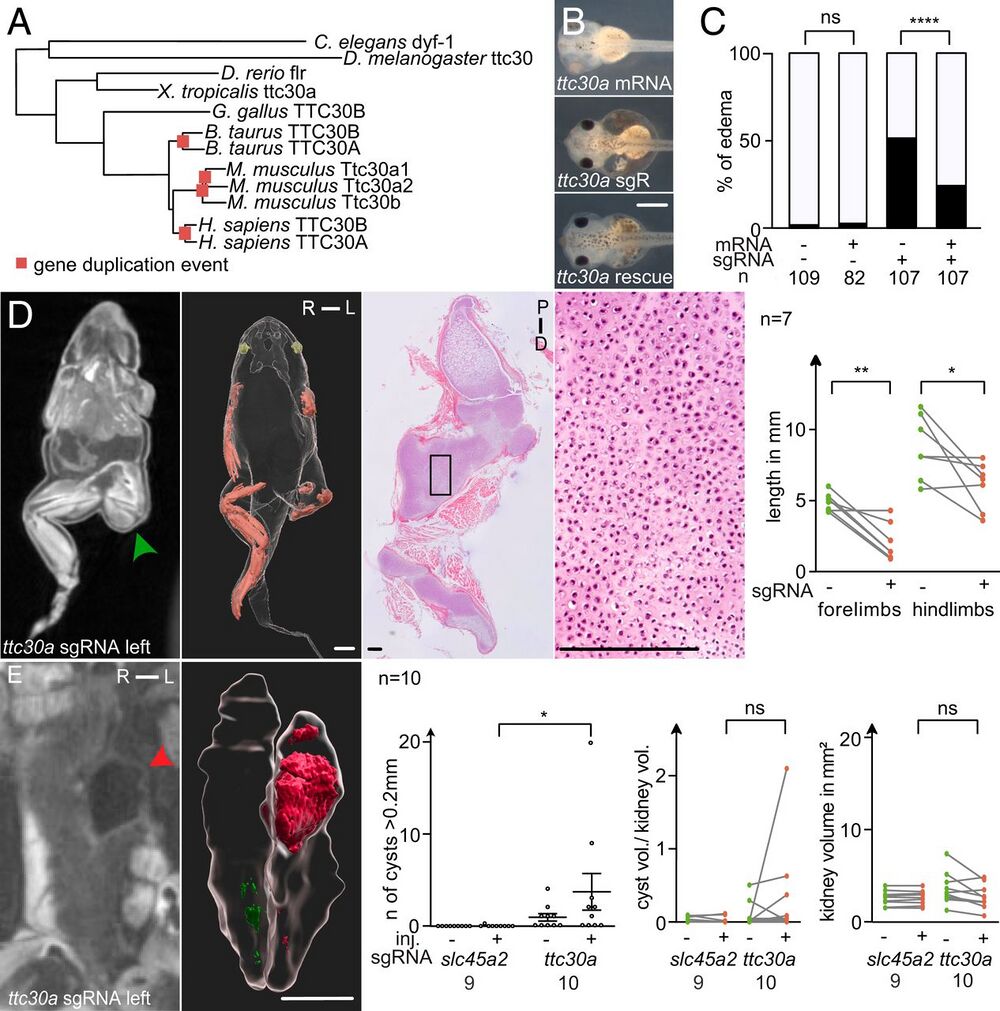
Fig. 4. ttc30 loss of function resembles the SC-phenotype. (A) Phylogenetic tree of protein sequences of TTC30A/B orthologs in various species. Genetic duplication events are marked by red squares. (B) CRISPR targeting of ttc30a at the one-cell stage led to edema formation in stage 41 tadpoles, quantified in (C). Coinjection of ttc30a mRNA partially rescued the phenotype. (D) Unilaterally CRISPR-targeted ttc30a froglets developed shortened limbs on the injected side (green arrowhead). MicroCT analysis, 3D reconstructions, and histological sections stained with hematoxylin-eosin demonstrated accumulation of cartilage (green arrowhead). Quantification of limb length reduction of ttc30a targeted compared with nontargeted side. (E) MicroCT scans show cystic kidneys in ttc30a-targeted animals (red arrowhead). 3D reconstruction of the kidneys and cysts (red) were used for volumetric analysis. Quantifications of cyst number, the ratio of total cyst volume to kidney volume, and total kidney volume (excluding cysts). [Scale bars, (B) 0.5 mm, (D and E, 3D reconstruction) 2 mm, (D, histological section) 200 µm.] R, right; L, left; P, proximal; D, distal. P > 0.05 ns (not significant); *P P P
Adapted with permission from National Academy of Sciences on behalf of PNAS: Getwan et al. (2021). Ttc30a affects tubulin modifications in a model for ciliary chondrodysplasia with polycystic kidney disease. PNAS September 28, 2021 118 (39) e2106770118; https://doi.org/10.1073/pnas.2106770118
