Developmental regulation of cellular metabolism is required for intestinal elongation and rotation
Grzymkowski JK, Chiu YC, Jima DD, Wyatt BH, Jayachandran S, Stutts WL, Nascone-Yoder NM.
Development 2024 Feb 15;1514:. doi: 10.1242/dev.202020.
Click here to view article at Development.
Click here to view article at Pubmed.
Click here to view article on Xenbase.
Intestinal malrotation is a very common birth defect—affecting as many as 1 in 500 births — but the etiology of this condition remains poorly understood. Malrotation can result from abnormal left-right patterning but is also often found independently of other laterality defects. In human patients, malrotation is associated with decreased intestinal length but the connection between gut rotation and gut elongation was relatively unexplored.
Grzymkowski et al. found that atrazine (ATR), a widely-used herbicide, causes reversed (clockwise) intestinal rotation in tadpole stage Xenopus, well after the left-right axis has been established and laterality cues have been conveyed to developing organs. Interestingly, ATR-induced malrotation is accompanied by disruption of the cellular events that drive gut tube elongation. Subsequent transcriptomic and metabolomic analyses suggest the herbicide causes these phenotypes by disrupting central carbon metabolism and inhibiting electron transport reactions - this prevents the gut from undergoing a metabolic shift from glycolysis-driven ATP production to mitochondrial oxidative phosphorylation. Consequently, ATR-induced malrotation elevates reactive oxygen species and, remarkably, can be rescued by treatment with an antioxidant, confirming that the mechanism of action involves redox imbalance.
Results from the Nascone-Yoder lab help explain the connection between gut elongation and rotation, and have implications for the role of metabolic perturbation in the etiology of intestinal malrotation. Their story also suggests there may be benefits to antioxidant supplementation during pregnancy.
Interesting write-up from Science Alert:
https://www.sciencealert.com/some-babies-are-born-with-twisted-insides-and-we-may-finally-know-why
Abstract
Malrotation of the intestine is a prevalent birth anomaly, the etiology of which remains poorly understood. Here, we show that late-stage exposure of Xenopus embryos to atrazine, a widely used herbicide that targets electron transport chain (ETC) reactions, elicits intestinal malrotation at high frequency. Interestingly, atrazine specifically inhibits the cellular morphogenetic events required for gut tube elongation, including cell rearrangement, differentiation and proliferation; insufficient gut lengthening consequently reorients the direction of intestine rotation. Transcriptome analyses of atrazine-exposed intestines reveal misexpression of genes associated with glycolysis and oxidative stress, and metabolomics shows that atrazine depletes key glycolytic and tricarboxylic acid cycle metabolites. Moreover, cellular bioenergetics assays indicate that atrazine blocks a crucial developmental transition from glycolytic ATP production toward oxidative phosphorylation. Atrazine-induced defects are phenocopied by rotenone, a known ETC Complex I inhibitor, accompanied by elevated reactive oxygen species, and rescued by antioxidant supplementation, suggesting that malrotation may be at least partly attributable to redox imbalance. These studies reveal roles for metabolism in gut morphogenesis and implicate defective gut tube elongation and/or metabolic perturbations in the etiology of intestinal malrotation.

Fig. 1. Exposure to atrazine causes intestinal shortening and malrotation. (A,C) Schematics illustrating normal counterclockwise (CCW) intestine rotation in wild-type (WT) Xenopus embryos (A), and the abnormal clockwise (CW) malrotation seen in ATR-exposed embryos (C). The midgut (future intestine) is yellow. Red (WT) and blue (ATR) arrowheads indicate the intestinal apex (NF 44, establishes the initial direction of rotation), and red (WT) and blue (ATR) spirals illustrate the final rotation direction of the intestinal coil (NF 46). (B,D) In situ stereo-microscope images of DMSO or ATR-treated NF 46 intestines (ventral view). DMSO control embryos develop elongated intestines that rotate normally (B), whereas ATR-exposed embryos develop intestine coils that are both short and malrotated (D). (E) The frequency of abnormal gut phenotypes increases with increasing concentrations of ATR, from predominantly normal (norm.) length (2+ intestine loops) and CCW rotation (rot.) to increasingly short (1.5 or fewer intestine loops) and/or CW malrotated (malrot.) configurations.
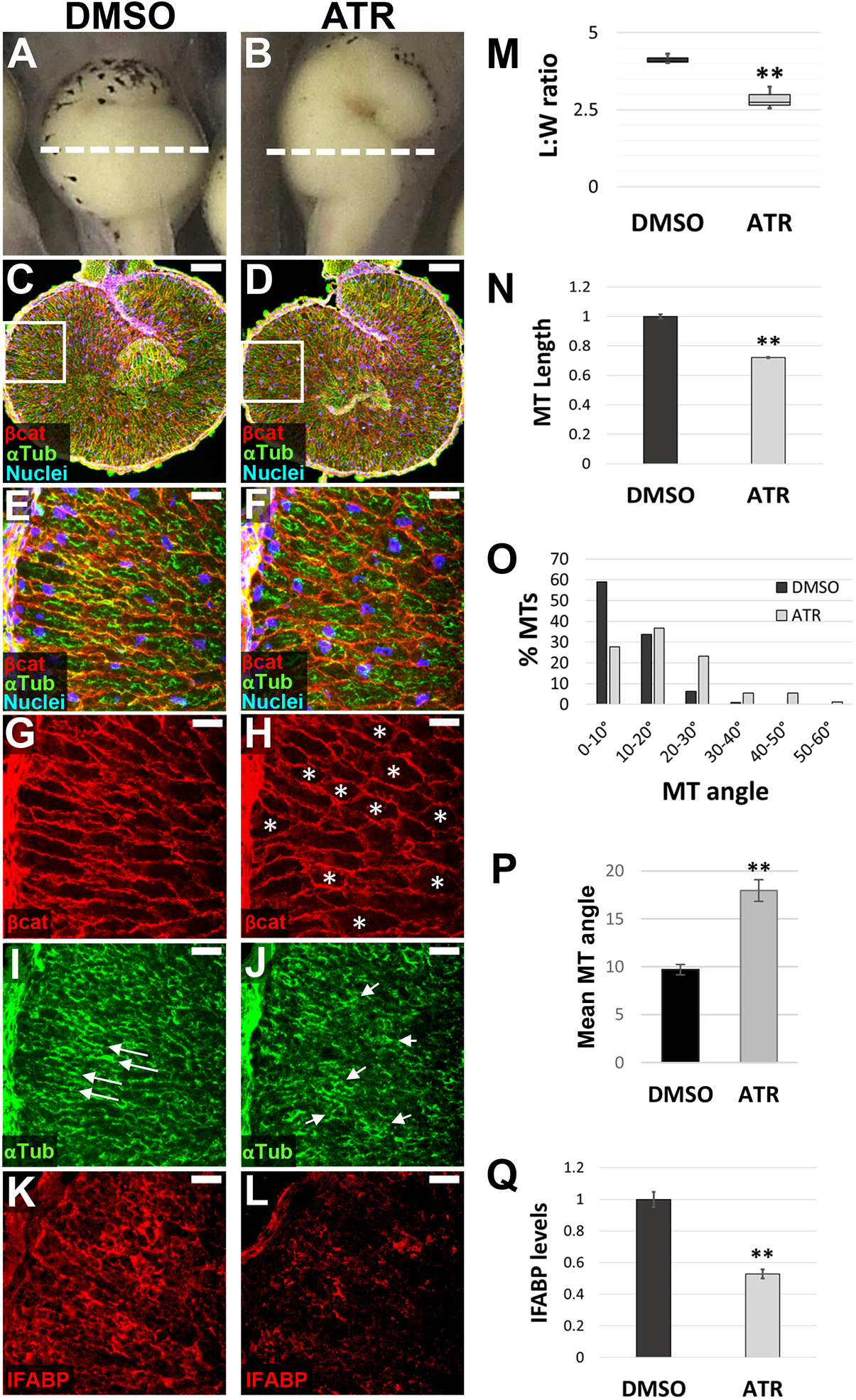
Fig. 2. ATR inhibits endoderm cell properties required for early intestine elongation. (A-Q) Transverse sections through the intestine of NF 42 control (DMSO; A,C,E,G,I,K) and ATR-exposed (B,D,F,H,J,L) embryos were immunostained for Beta-catenin (red; C-H) to outline cell membranes, alpha-tubulin (green; C-F,I,J) to visualize MT bundles, and IFABP (red; K,L) to mark differentiated intestinal epithelial cells. Nuclei (TO-PRO-3) are blue. Dashed lines in A,B indicate the approximate location of DMSO and ATR sections. Boxed regions in C and D are shown at higher magnification in E,G,I and F,H,J, respectively, and approximate the locations of K and L, respectively, in neighboring sections. Note that the control image shown in C is the same as that displayed in Fig. 6A. Cells of ATR-exposed intestines are rounder in shape (G,H, asterisks), as indicated by decreased L:W ratios of individual cells (M), and have short (N), misoriented MT bundles (I,J, arrows; quantified in O,P) and low levels of IFABP (K,L,Q), compared with DMSO controls. Error bars represent s.e.m. **P<0.01 (two-sample t-test). Scale bars: 100um (C,D); 25um (E-L).
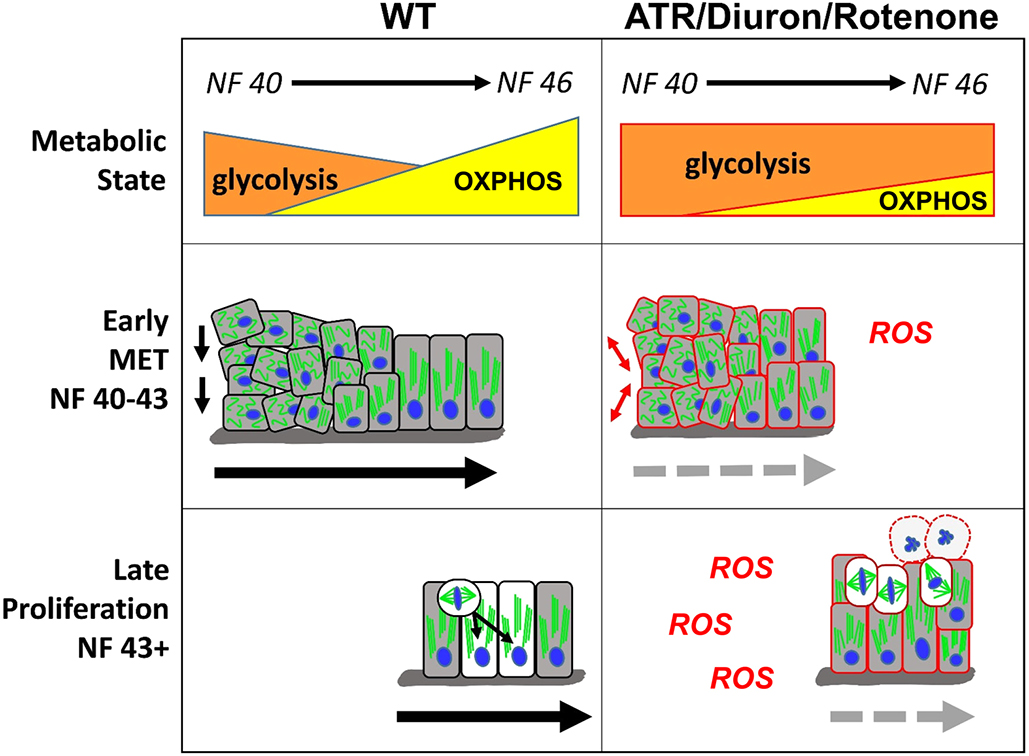
Fig. 9. Model for how cellular metabolism affects gut elongation. Left: At early stages of gut morphogenesis (NF 40-43), cell rearrangements in wild-type (WT) embryos are promoted by a primarily glycolytic metabolism. Gradually, as a single-layer epithelium is established, increasing OXPHOS supports the completion of MET and intestine cell differentiation. At late stages (NF 43+), the more energy-efficient mitochondrial (OXPHOS) metabolism is also required to support increased rates of proliferation and INM. Right: Inhibition of mitochondrial ETC function by metabolic perturbagens (ATR/Diuron/rotenone) decreases OXPHOS, elevates ROS and prolongs glycolytic activity, thereby preventing the completion of MET and retaining a disorganized epithelium of undifferentiated cells. Continued inhibition of OXPHOS and consequent oxidative stress at later stages retains the primarily glycolytic metabolic state, leading to mitotic arrest and eventual apoptosis (round cells with dashed outlines). Combined, the perturbation of both early (MET) and late (proliferation) elongation processes results in short intestines that fail to achieve the length necessary to undergo proper rotation.
Adapted with permission from The Company of Biologists on behalf of Development: Grzymkowski. (2024). Developmental regulation of cellular metabolism is required for intestinal elongation and rotation. Development 2024 Feb 15;1514:. doi: 10.1242/dev.202020.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2024-03-13