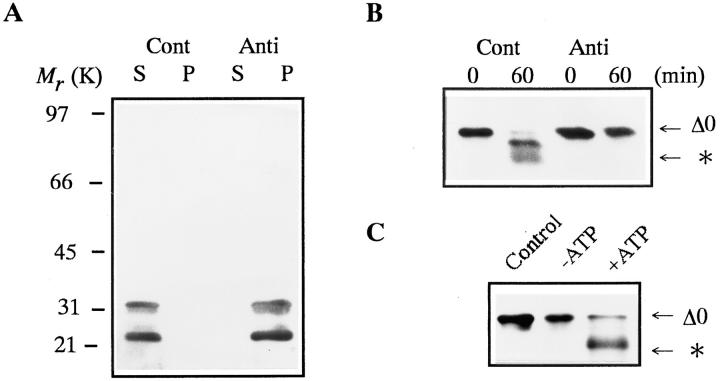
Figure 2. Inhibition of cyclin B digestion by proteasome or ATP depletion. Cyclin B was detected by the B63 antibody. The position to which the digested cyclin B migrated is indicated by an asterisk. (A) Immunodepletion of 26S proteasome from purified 26S proteasome fraction. 26S Proteasome was immunoprecipitated by affinity-purified anti-proteasome IgG (Anti) or control IgG (Cont), as described (Tokumoto and Ishikawa, 1993). Supernatants (S) and precipitates (P) were immunoblotted with a mixture of three monoclonal antibodies against goldfish 20S proteasome (GC4/5, 3α and 3β; Tokumoto et al., 1995a). (B) Digestion of cyclin B in the mock- (Cont) or proteasome-depleted (Anti) 26S proteasome fraction. Cyclin Δ0 (5 μg/ml) was incubated at room temperature with the supernatant after immunoprecipitation with control or anti-proteasome IgG. Samples were exposed to Laemmli's SDS sample buffer at the indicated times during incubation. (C) Effect of ATP depletion on cyclin B digestion by the 26S proteasome. Cyclin Δ0 (5 μg/ml) was incubated at room temperature for 60 min without (Control) or with the 26S proteasome in the presence of an ATP-depleting system (10 mM glucose and 1 μg/ml hexokinase, −ATP) or 2 mM ATP (+ATP).
Image published in: Tokumoto T et al. (1997)
Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution-NonCommercial-ShareAlike license
Permanent Image Page
Printer Friendly View
XB-IMG-117539