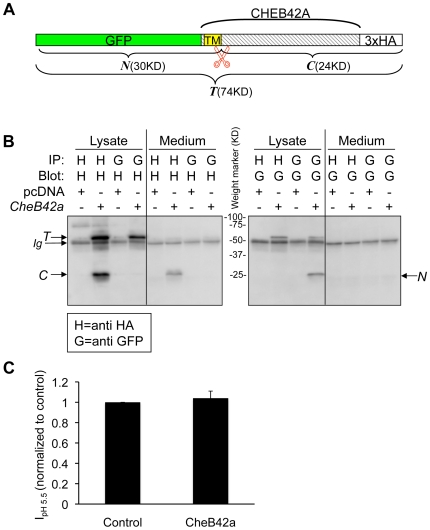
Figure 7. CHEB42A produces a secreted peptide.A. Schematic of the construct used to study CHEB42A. CheB42a cDNA was tagged with GFP at the 5′ end and 3xHA tags at the 3′ end and was cloned into the mammalian expression vector pcDNA3.1 (Invitrogen). TM, transmembrane peptide as predicted by the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/). predicted transmembrane domain. Predicted signal peptide cleavage site. T, N, and C represent the predicted full length and cleaved products. B. CHEB42A is a secreted protein. Cells were transfected with tagged cDNA (CheB42a) or pcDNA3.1 as a control. Proteins from cell lysates or cell media were immunoprecipitated (IP) with either anti-GFP (G) or anti-HA (H) antibodies, and then blotted for either tag. T, N, and C indicate the predicted peptides from Fig. 7A. C. Effects of CheB42a-conditioned media on ASIC1a currents. ASIC1a was expressed in Xenopus oocytes as in Fig. 6. Four ASIC1a-expressing oocytes were tested for pH-dependent currents with and without conditioned media from CheB42a-expressing cells. The same oocytes (N = 4) were stimulated with unconditioned medium (pH 5.5), followed by stimulation with CheB42a-conditioned medium. There was no obvious effect of the conditioned media on pH-activation of ASIC1a channels. Currents were normalized in the conditioned state relative to the control media. All oocytes showed significant ASIC1a-dependent currents in response to pH 5.5, which does not elicit any currents in non-injected oocytes.
Image published in: Ben-Shahar Y et al. (2010)
Ben-Shahar et al. Creative Commons Attribution license
Permanent Image Page
Printer Friendly View
XB-IMG-124712