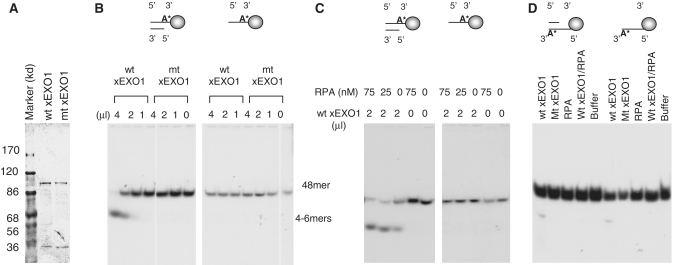
Figure 2. Characterization of xEXO1's nuclease activity. (A) A SDS–PAGE showing the purified recombinant wild-type and mutant xEXO1. (B) Nuclease assay of recombinant xEXO1 with different DNA substrates. The substrates were 48-mer oligonucleotides in either ss- or ds-form attached to magnetic beads, leaving the 5′-end (of the 32P-labeled strand) accessible to the nuclease. After 1 h of incubation at room temperature, the reactions were terminated with 1% SDS, heated at 95°C for 15 min, and then separated on an 8% TAE–PAGE. (C) Effect of RPA on the 5′→3′ exonuclease activity of xEXO1. Different amounts of RPA and xEXO1 were incubated with the 32P-labeled 5′ accessible ss- or ds-48-mer beads for one hour at room temperature. The analysis was similar to that in (A). (D) Determination of the 3′→5′ exonuclease activity of xEXO1. Four microliters of xEXO1 was incubated with 32P-labeled 3′ accessible ss- or ds-48-mer beads in the presence or absence of RPA (75 nM) at room temperature for 1 h. The analysis was similar to that in (A).
Image published in: Liao S et al. (2011)
© The Author(s) 2011. Creative Commons Attribution-NonCommercial license
Permanent Image Page
Printer Friendly View
XB-IMG-126292