XB-IMG-159445
Xenbase Image ID: 159445
|
|
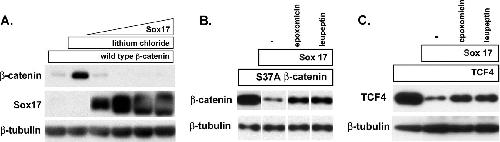
Figure 8. Sox17-mediated degradation of β-catenin and TCF4 is independent of GSK3β activity and mediated by the proteasome. (A) LiCl does not affect Sox17-mediated degradation of β-catenin. COS cells were transfected with an expression plasmid encoding wild-type β-catenin (100 ng; myc tagged) in the absence (lane 1) or presence(lanes 2 to 6) of 40 mM LiCl. Cells were cotransfected with increasing amounts of Sox17 plasmid (50, 100, 200, and 400 ng) and the wild-type β-catenin gene in the presence of lithium chloride (lanes 3 to 6). LiCl effectively stabilized wild-type β-catenin protein in the absence of Sox17 but could not inhibit Sox17-mediated degradation of wild-type β-catenin protein. (B and C) The proteasome inhibitor epoxomicin inhibits Sox17-induced degradation of stabilized β-catenin (B) and TCF4 (C). Cells were transfected with the β-catenin(S37A) mutant form (100 ng) or TCF4 (100 ng) alone or together with Sox17 (100 ng). Culturing cotransfected cells with epoxomicin (0.1 μM) or leupeptin (25 μM) inhibited Sox17-mediated degradation of β-catenin and TCF4 proteins. The lysosomal acidification inhibitor cholorquine (25 μM) had no effect (data not shown). â, control. Image published in: Sinner D et al. (2007) Copyright © 2007. Image reproduced with permission of the Publisher, American Society for Cell Biology (ASCB). This is an Open Access article distributed under the terms of the Creative Commons Attribution License. Larger Image Printer Friendly View |
