XB-IMG-127891
Xenbase Image ID: 127891
|
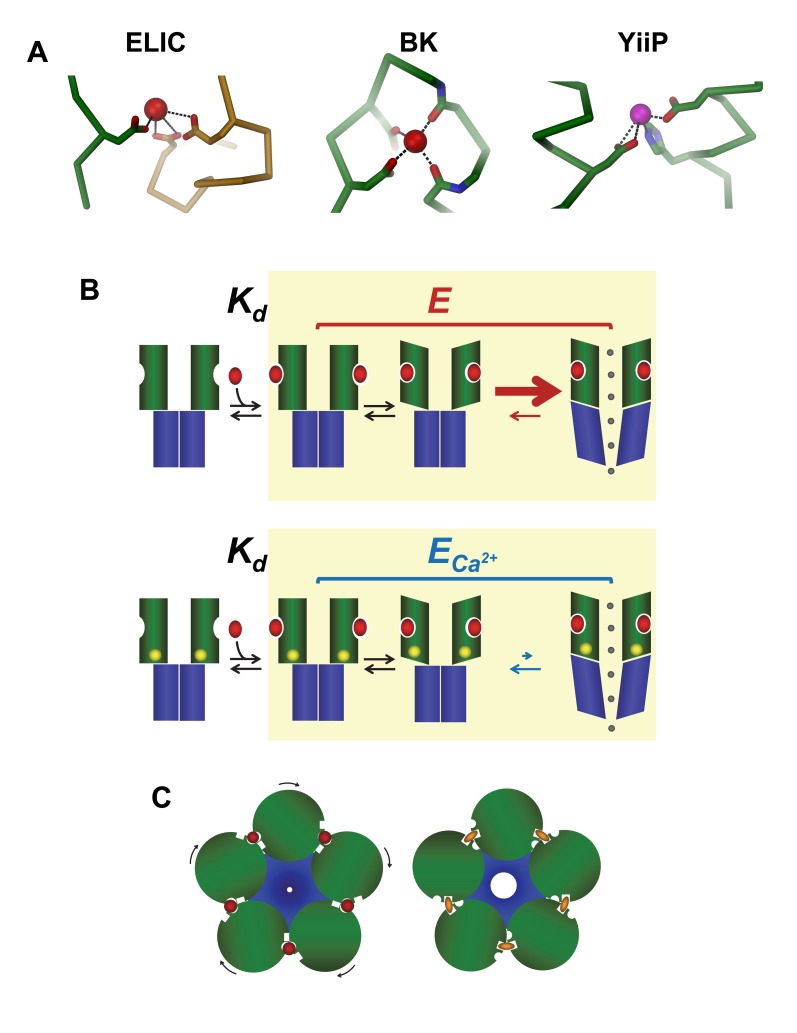
Figure 7. Potential mechanisms.(A) Interactions in the regulatory divalent ion-binding site of ELIC (left) in comparison with a regulatory Ca2+ binding site of the BK-channel (middle) and the Zn2+-transporter YiiP (right). (B) Schematic model of a potential mechanism for the inhibitory effect of divalent ions. The two rows show simplified schemes for channel activation in control conditions (top) and in the presence of divalent ions. From left to right, the schemes show that binding of agonist molecules (red ovals) to the extracellular domain (with microscopic affinity Kd) is followed by conformational changes (yellow background) that result in channel opening. Channel gating (described by the efficacy equilibrium constant E) is impaired when the channel is bound to divalent ions (yellow circles, ECa2+). The decrease in agonist efficacy is likely to be due to a change in the rate of opening, as shown by the size of the arrows in the last step of the reaction. (C) Schematic mechanism of how binding sites located on similar places of an oligomeric channel could alternately stabilize the closed or open conformation of the channel. Image published in: Zimmermann I et al. (2012) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution license Larger Image Printer Friendly View |
