XB-IMG-122343
Xenbase Image ID: 122343
|
|
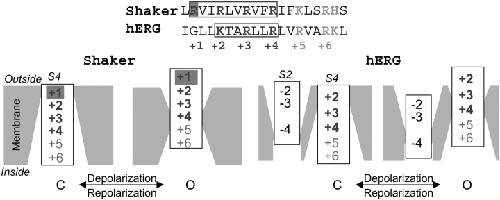
Figure 10. . (Top) Alignment of S4 amino acid sequences of Shaker and hERG. The positive charges are denoted by generic numbers below. Boxes highlight voltage-sensing (gating) charges in each channel. Gray shade highlights the Shaker arginine that is absent in hERG. (Bottom) Diagrams illustrating putative movements of Shaker's S4 and hERG's S2 and S4, relative to the membrane barrier, during channel activation. The Shaker diagram is based on (Starace and Bezanilla, 2004), with the first four positive charges switched from internally exposed positions in closed states to externally exposed in the open states. Although E293 in the Shaker S2 may contribute to gating charge transfer, it is not included in the diagram because there is no data on the accessibility of 293C to MTSETo or MTSETi. The hERG diagram is based on data from the present study, with one exception. Although we cannot detect any MTSETo accessibility of 528C (+3 in the diagram) in the open state, this side chain is accessible to external p-chloromercuibenzene sulfonate (pCMB, a smaller thiol-modifying reagent than MTSET) in the open state (Mitcheson, J.S., personal communication). The crevice around S2 is deep in the hERG channel, so that cysteine side chain at position −3 is readily accessible to extracellular MTSETo (Liu et al., 2003). Cysteine at position −4 (466C) is accessible to MTSETo in the closed state but not in the open state. MTSETi also modifies 466C, although the effect is much less than that of MTSETo in the closed state, and there is no clear state dependence in MTSETi modification of 466C. These observation are depicted by a narrow inwardly accessible crevice around S2 at the −4 level in both closed and open states. Image published in: Zhang M et al. (2004) Copyright © 2004, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
