XB-IMG-136738
Xenbase Image ID: 136738
|
|
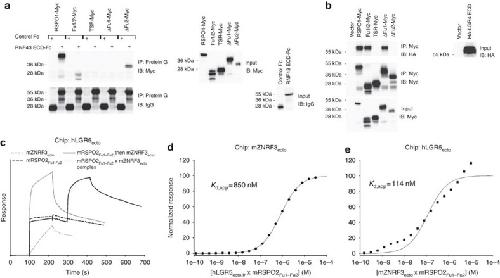
Figure 6. The LGR–Rspo–ZNRF3/RNF43 ternary complex.(a) RNF43 interacts with Fu1 of human Rspo1. The secreted RNF43 ectodomain co-immunoprecipitated Rspo1 and its derivatives, Rspo1Fu1–Fu2 and Rspo1ΔFu2, but neither RSPO1ΔFu1 nor Rspo1TSR in conditioned media (CM; left). RNF43 is IgG-tagged, whereas Rspo1 and derivatives are Myc-tagged, and their secretion levels in CM were also examined (right). (b) LGR4 interacts with Fu2 of Rspo1. The secreted LGR4 ectodomain was co-immunoprecipitated by Rspo1 and its derivatives, Rspo1Fu1–Fu2 and Rspo1ΔFu1, but by neither Rspo1ΔFu2 nor Rspo1TSR in CM (left). Secreted LGR4 is HA-tagged and its secretion in CM was examined as were Rspo1 and derivatives (right). (c) Step-by-step ternary complex assimilation. hLGR5ecto (R32-G557) was immobilized on an SPR chip, followed by injections of 10 μM solutions of mZNRF3ecto, mRSPO2Fu1–Fu2, mRSPO2Fu1–Fu2 followed by mZNRF3ecto or a preformed mZNRF3ecto × mRSPO2Fu1–Fu2 complex. mZNRF3ecto shows some direct interaction with LGR5 characterized by a slow on-rate (thin dashed line). Binding is much faster if LGR5 is first saturated with mRSPO2 Fu1–Fu2 (thick solid line). Similar responses are observed when a 1:1 complex of mRSPO2Fu1–Fu2 × mZNRF3ecto is injected. (d) Saturation of immobilized mZNRF3ecto with the hLGR5ecto × mRSPO2Fu1–Fu2 complex that was stable in gel filtration. lr, loop removed: A488-H537→NGNNGD. (e) Saturation of immobilized hLGR5ecto with the mZNRF3ecto × mRSPO2Fu1–Fu2 complex that was stable in gel filtration. Single dilution series. Image published in: Zebisch M et al. (2013) Copyright © 2013, Nature Publishing Group, a division of Macmillan Publishers Limited. Creative Commons Attribution license Larger Image Printer Friendly View |
