XB-IMG-118909
Xenbase Image ID: 118909
|
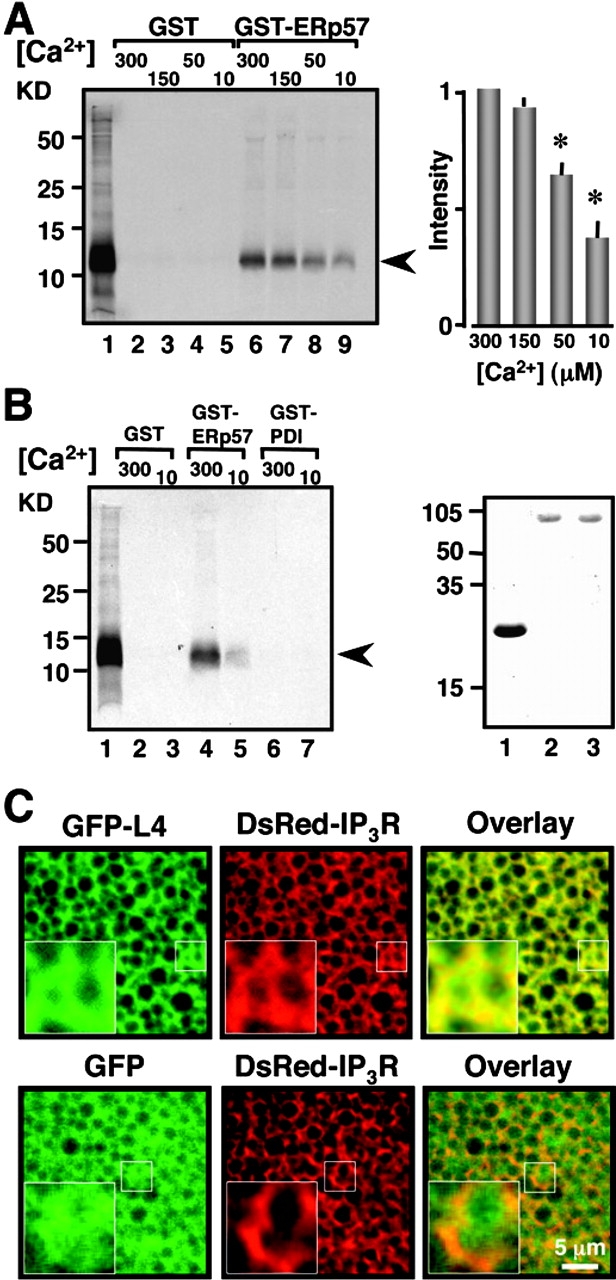
Figure 5. The interaction between ERp57 and L4 is Ca2+ dependent and specific. (A) GST pull-down assays performed at the indicated [Ca2+] in micromolars. The gel is loaded as follows: input of in vitro–translated L4 (lane 1); pull downs for 30 μg GST control (lanes 2–5); pull downs for 10 μg GST-ERp57 (lanes 6–9). Proteins were resolved through 15% SDS-PAGE. L4 migrates ∼11 kD (109 aa). Data represent three independent experiments. Histogram depicts densitometric analysis from these experiments. L4 bands were normalized to the intensity of the band at 300 μM Ca2+. Asterisks indicate statistical significance (P < 0.05, one-way ANOVA). (B) The interaction between ERp57 and L4 is specific by GST pull-down assay. Lanes were loaded as follows: input of in vitro–translated L4 (lane 1); 30 μg GST negative control at the indicated [Ca2+] (lanes 2 and 3); 10 μg GST-ERp57 (lanes 4 and 5); 10 μg GST-PDI (lanes 6 and 7). Proteins were resolved through 15% SDS-PAGE. The gel represents three independent experiments. (Right gel) Coomassie blue stain of 3 μg purified GST (lane 1); 1 μg GST-ERp57 (lane 2), and 1 μg GST-PDI (lane 3). Proteins were resolved through 13% SDS-PAGE. GST migrates ∼27 kD, and GST-ERp57 and GST-PDI migrate ∼80 kD (mature rPDI is 489 aa, mature hERp57 is 481 aa). The arrowheads correspond to L4. (C) The L4 protein localizes to the ER. (Top) Coexpression in Xenopus oocytes of GFP-L4 (green) with DsRed-IP3R (red). The IP3R is used as a marker of ER localization. The overlay (yellow) demonstrates that the L4 localizes in the ER. (Bottom) Cytosolic GFP (green) used a negative control, is coexpressed with DsRed-IP3R (red). Note that the cytosolic GFP expression pattern is more diffused than that of the GFP-L4 and that there is no overlap of the two proteins indicating lack of colocalization. High magnification of the small square regions are shown as insets in each figure. Bar, 5 μm. Image published in: Li Y and Camacho P (2004) Copyright © 2004, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
