XB-IMG-125817
Xenbase Image ID: 125817
|
|
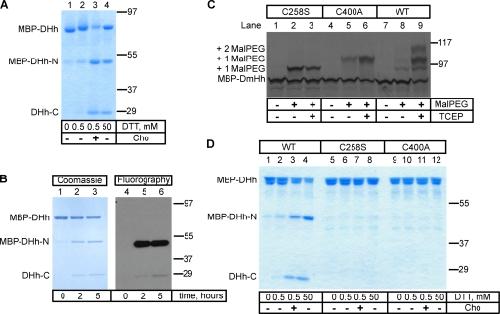
Figure 1. Processing of the purified Hh precursor. (A) A fusion was generated between maltose-binding protein (MBP), the last 15 amino acids of the N-terminal fragment of Drosophila Hh (DHh), and the entire C-terminal fragment of DHh (MBP-DHh). The purified protein was incubated for 5 h at room temperature with different concentrations of DTT in the absence or presence of cholesterol (Cho). The samples were analyzed by nonreducing SDS-PAGE and Coomassie staining. MBP-DHh–N and DHh-C are the N- and C-terminal fragments generated by Hh processing. (B) As in A, but the reaction contained 3H-labeled cholesterol. The samples were analyzed by reducing SDS-PAGE followed by either Coomassie staining or fluorography. (C) In vitro translated 35S-labeled wild-type (WT) MBP-DHh or the indicated cysteine mutants were incubated with 5-kD maleimide-polyethylene glycol (Mal-PEG) as indicated, in the presence or absence of the reducing agent tris(2-carboxyethyl)phosphine (TCEP). The samples were analyzed by reducing SDS-PAGE and autoradiography. The positions of singly and doubly Mal-PEG–modified species are indicated. The singly modified species have a different mobility, depending on which cysteine is modified. (D) As in A, but comparing wild-type MBP-DHh with the two cysteine mutants. Molecular masses are given in kilodaltons. Image published in: Chen X et al. (2011) © 2011 Chen et al. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
