XB-IMG-123680
Xenbase Image ID: 123680
|
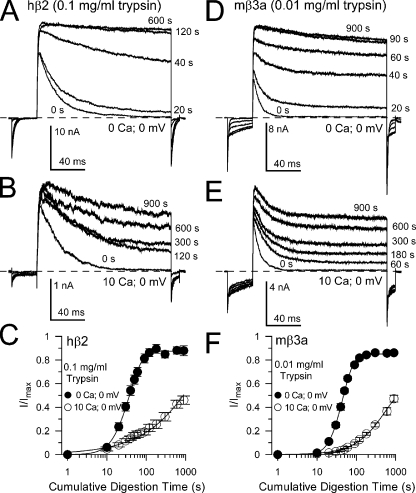
Figure 6. Digestion of mβ3a N termini is much faster than for β2. (A) Traces show removal of hβ2-mediated inactivation under closed-channel conditions (0 Ca2+; 0 mV) with 0.1 mg/ml trypsin. (B) Traces show removal of β2-mediated inactivation by 0.1 mg/ml trypsin under inactivating conditions (10 µM Ca2+; 0 mV). (C) The digestion time course is plotted for both closed (solid circles) and inactivating (open circles) hβ2 channels. (D) Sweeps monitor removal of mβ3a-mediated inactivation under closed-channel conditions, but with 0.01 mg/ml trypsin. Trypsin abolishes both the slow β3a tail currents and removes inactivation. (E) The time course of removal of mβ3a-mediated inactivation by 0.01 mg/ml trypsin is shown under inactivating conditions. Note that even at 900 s, appreciable inactivation still remains and that the slow β3a tail current persists. (F) The digestion time course for mβ3a channels is plotted for both closed-channel (solid circles) and inactivating (open circles) conditions. Image published in: Zhang Z et al. (2009) © 2009 Zhang et al. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
