XB-IMG-125154
Xenbase Image ID: 125154
|
|
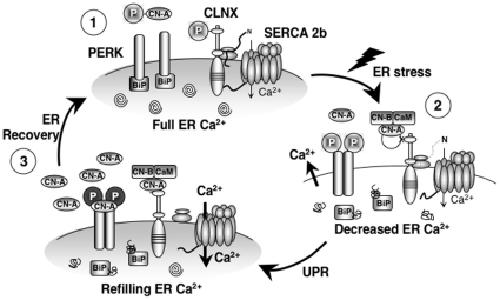
Figure 8. Role of CN in the early phases of ER stress.(1) Resting conditions of the ER: CLNX is phosphorylated, interacting with SERCA 2b and inhibiting its activity. CLNX is also interacting with the ribosome, increasing the capacity of protein folding. PERK is associated with BiP, which prevents its autophosphorylation. Protein processing and folding is optimal (depicted by spirals). (2) ER stress: unfolded proteins accumulate in the ER lumen, BiP dissociates from PERK, permitting its dimerization and autophosphorylation, which leads to attenuation of protein synthesis. At the same time, Ca2+ is released, activating CN, inducing dephosphorylation of CLNX, thereby removing pump inhibition. (3) CN levels are increased, leading to the association of CN with pre-activated PERK, which induces further PERK auto-phosphorylation, increasing the phosphorylation level of eIF2α. This emphasizes the protein translation inhibition. If cell Ca2+ levels are restored (1), CN becomes phosphorylated by PERK, decreasing its activity. CN expression also returns to resting levels further reducing its signaling. These steps, in combination with a full Ca2+ store and BiP re-association with PERK, restore normal protein translation and ER homeostasis. Image published in: Bollo M et al. (2010) Bollo et al. Creative Commons Attribution license Larger Image Printer Friendly View |
