XB-IMG-117805
Xenbase Image ID: 117805
|
|
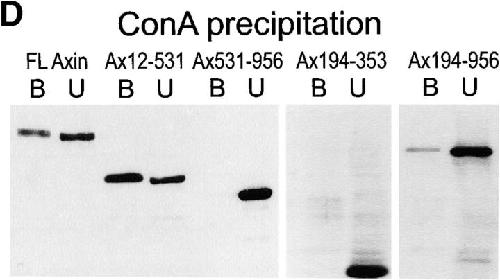
Figure 8. Cell fractionation of Myc–Axin in Xenopus embryos: sedimentability and Con A binding. (A) Diagram of Axin molecule. (B) Myc–Axin is associated with a sedimentable fraction. Homogenates of late blastula embryos were fractionated into a low speed sedimentable fraction (P, pellet), a high speed sedimentable fraction (M, membranes), and a high speed supernatant (S, soluble fraction) as described in Materials and Methods. β-Galactosidase was coexpressed and used as a control for soluble cytosolic proteins. Unlike β-galactosidase, Myc–Axin was sedimentable under these conditions. (C) Myc–Axin is fully extractable in NP-40. Embryos expressing Myc–Axin were extracted in NP-40–containing buffer (sol). The insoluble pellet was reextracted in the presence of SDS (insol, NP-40– insoluble). (D) A pool of Axin is associated with a membrane glycoprotein, and this association requires the NH2-terminal domain. Myc-tagged FL Axin and various Axin mutant constructs were expressed in embryos, and NP-40 extracts were fractionated using Con A beads. Bound fractions (B) were four times concentrated relative to unbound fractions (U). FL Axin showed significant association with Con A beads that indicates a stable interaction with a membrane glycoprotein. Stronger binding was observed for the NH2-terminal fragments Ax12-531. On the other hand, constructs lacking the NH2-terminal domain showed no (Ax-531-956 and Ax194-353) or very weak binding (Ax194-956). Image published in: Fagotto F et al. (1999) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
