XB-IMG-122478
Xenbase Image ID: 122478
|
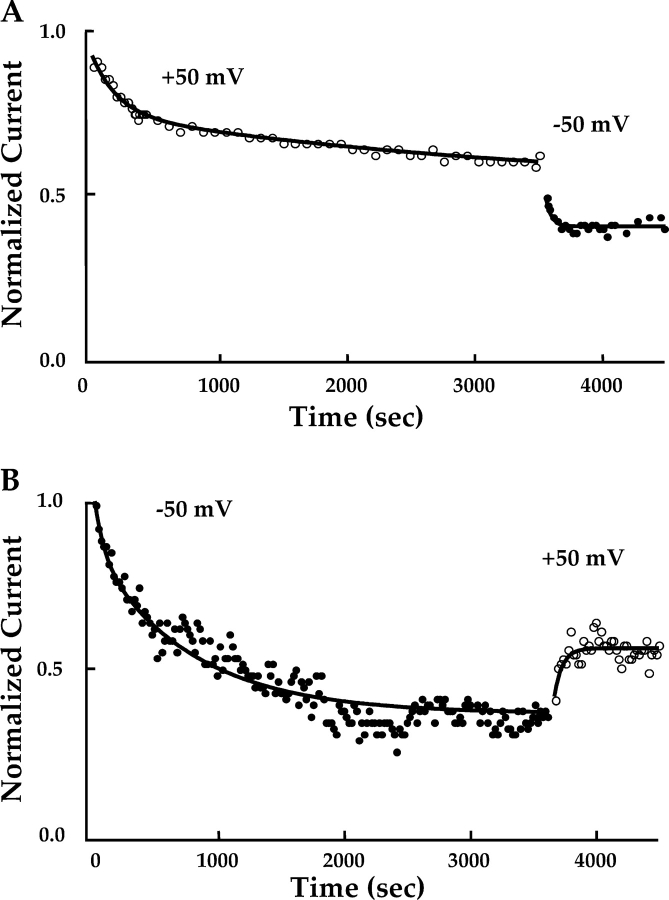
Figure 7. . Inhibition of homomeric (CNGA1) rod channels by 200 nM ATR in saturating (2 mM) cGMP showed no significant voltage dependence other than that expected with voltage-dependent changes in open probability. For the top panel, an inside-out, multichannel patch was maintained at a holding potential of +50 mV in 200 nM ATR and 2 mM cGMP and monitored to steady-state. A double exponential provided the best fit to the time course of the inhibition, with τfast = 206 s and τslow = 3,779 s. After steady-state was achieved, the holding potential was changed to −50 mV. The time course of the resulting decrease in current (increase in inhibition) was best fit with a single exponential, with τ = 44 s. For the lower panel, the same experiment was performed on another patch, except that the initial membrane potential was −50 mV, and it was then switched to +50 mV. A double exponential provided the best fit to the initial time course of inhibition, with τfast = 77 s and τslow = 769 s. The time course of the increase in current (decrease in inhibition) after switching the voltage to +50 mV was best fit with a single exponential, with τ = 64 s. These changes in ATR inhibition with voltage are consistent with the expected voltage-dependent changes in open probability and the greater ATR inhibition of closed versus open channels. Image published in: McCabe SL et al. (2004) Copyright © 2004, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
