XB-IMG-117310
Xenbase Image ID: 117310
|
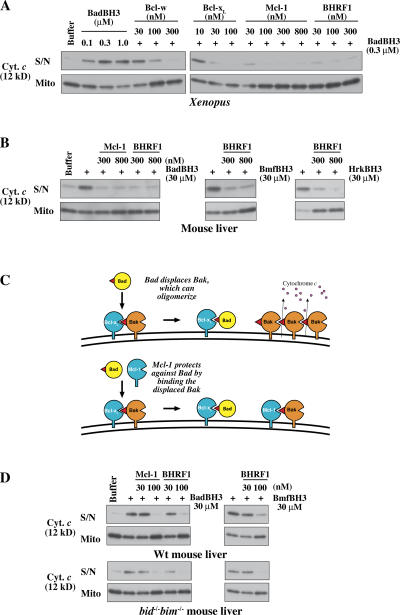
Figure 4. Mcl-1 and BHRF1 can even block the action of those BH3 peptides that they cannot bind. (A) Permeability of XEM induced by BadBH3 is efficiently blocked by prosurvival proteins that cannot bind Bad. XEM were preincubated for 20 min with the indicated concentrations of Mcl-1ΔC11 or BHRF1ΔC16 and were supplemented with mBadBH3 peptide for a further 2 h. Supernatant and pellets were then examined for cytochrome c by Western blotting. Similar experiments involving Mcl-1 and BHRF1-mediated protection from hBad, Bmf, Hrk, Bim, and Puma BH3 peptides are shown in Fig. S4 (available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1. (B) Permeability of MLM induced by mBad, Bmf, and Hrk BH3 peptides is efficiently blocked by Mcl-1 and BHRF1. MLM were incubated as in A with the indicated combinations of prosurvival proteins and BH3 peptides. (C) Model of how MLM are permeabilized by Bad (top) and protected by Mcl-1 (bottom). For simplicity, all of the Bak in the healthy cell is depicted as bound to Bcl-xL, but the indirect activation model postulates that only a proportion of the Bak molecules is in that form. (D) Permeabilization of mitochondria lacking both Bim and Bid by the sensitizer BH3 peptides Bad and Bmf. In an experiment like that in B, the absence of both of these putative activators does not prevent cytochrome c release or affect the protection conveyed by Mcl-1 and BHRF1, which do not bind Bad or Bmf. S/N, supernatant; Mito, mitochondrial pellet; Wt, wild type. Image published in: Uren RT et al. (2007) Copyright © 2007, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
