XB-IMG-171113
Xenbase Image ID: 171113
|
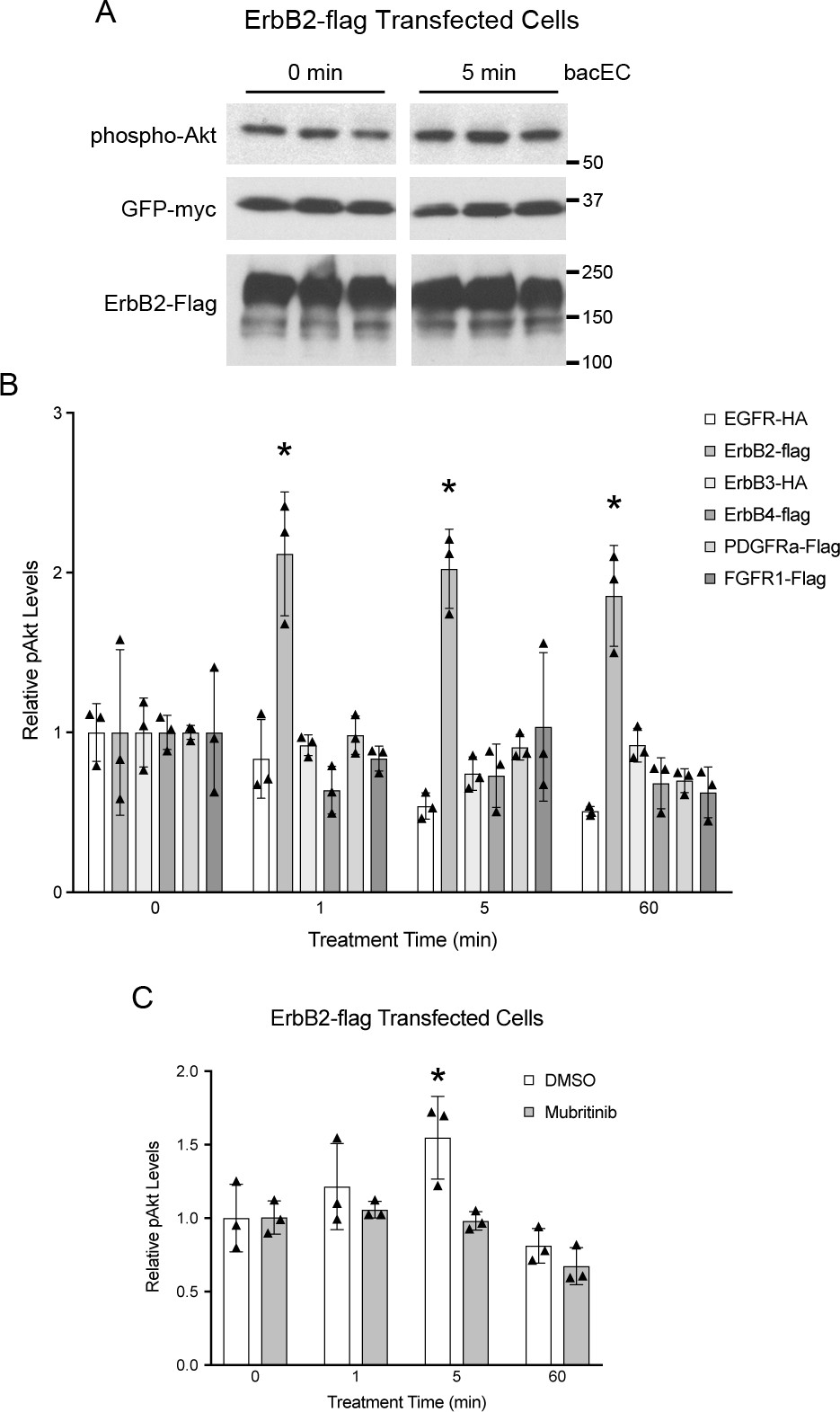
Fig 4. Recombinant EC1-3 (bacEC) increases Akt phosphorylation in ErbB2-transfected cells.
Hek293T cells were transfected with receptor tyrosine kinases, serum-starved and treated with bacEC for indicated lengths of time. Lysates were probed for phospho-Akt (pAkt) and GFP-myc. The latter was co-transfected with receptor constructs to account for variation in transfection efficiency that could result in changes to receptor protein levels. (A) Representative western blot of lysates from ErbB2-transfected cells (in triplicate). (B,C) Quantification of pAkt from cell lysates. GFP-myc was used for normalization. (B) ErbB2-expressing cells had significantly higher pAkt levels after exposure to bacEC (p = 0.018â0.036). The p values are p = 0.02, p = 0.018 and p = 0.036 for the 1, 5, and 60 minute time points. (C) Addition of 600 nM mubritinib to ErbB2-transfected cells abrogated the effect bacEC on Akt phosphorylation. Results are representative of three independent experiments. Means were calculated from triplicates and shown as bars. Data points are shown as stars. One-tailed, Studentâs t-tests were performed to determine statistical significance. * p<0.05.
https://doi.org/10.1371/journal.pone.0188963.g004 Image published in: Mathavan K et al. (2017) © 2017 Mathavan et al. Creative Commons Attribution license Larger Image Printer Friendly View |
