XB-IMG-118569
Xenbase Image ID: 118569
|
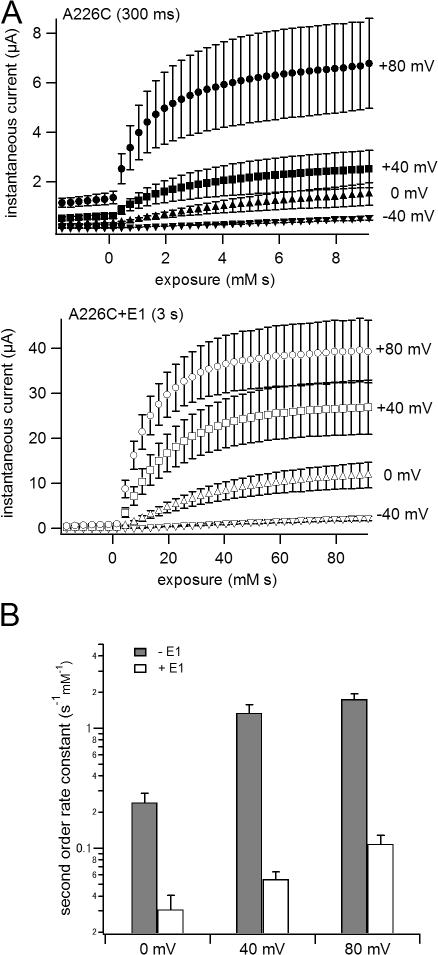
Figure 4. MTS reaction rate is voltage dependent. (A) Time courses of MTSES reaction with A226C (300 ms) and A226C+KCNE1 (3 s) with depolarization to −40, 0, +40, and +80 mV are shown. (B) Apparent second order rate constants are plotted against voltage. Although time courses of the reaction with depolarizations to +40 and +80 mV were fitted with double exponential function, only the faster time constants were used for the calculation of the apparent second order rate constants. Filled bars represent rate constants without KCNE1, open bars the rate constants with KCNE1. Image published in: Nakajo K and Kubo Y (2007) Copyright © 2007, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
