XB-IMG-126871
Xenbase Image ID: 126871
|
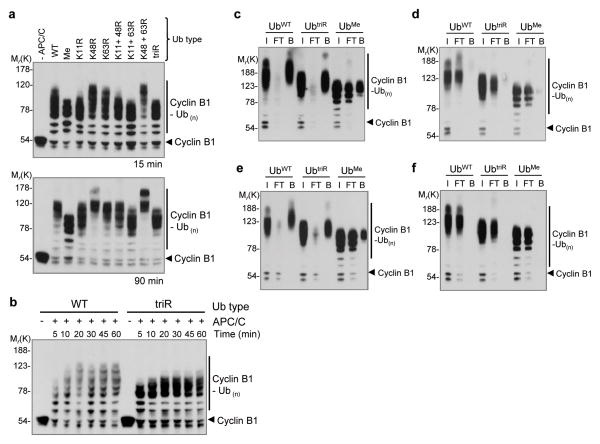
Figure 4. UBCH10 and APC/C catalyze rapid multiple monoubiquitination of cyclin B1 that is sufficient for binding ubiquitin receptors. (a) Western analysis of in vitro ubiquitination reaction containing full-length cyclin B1, APC/C immunopurified from mitotically-arrested Xenopus extract, recombinant UBCH10 (100 nM), and forms of Ub (118 μM), as indicated. Ubiquitin types with lysine-to-arginine mutations at one, two, or at all three positions Lys11, 48 and 63 (UbtriR), as well as methylated ubiquitin (Ubme) were used. Control “−APC/C” reactions containing all components except for the E3 ligase were performed in parallel. Reactions were allowed to proceed for 15 or 90 min before analysis by SDS-PAGE/western blotting against cyclin B1. (b) Time-course of the in vitro ubiquitination of full-length wild-type cyclin B1 with UbWT or UbtriR and remaining components as in a. (c–f) Binding of ubiquitinated cyclin B1 to GST-tagged Ub receptors. Cyclin B1-Ub conjugates were incubated with immobilized receptor proteins for 1 h at 4 °C before reaction products were subjected to SDS-PAGE/western blot analysis against cyclin B1. Equivalent amounts of input (I), flow-through (FT) and bound (B) were loaded in adjacent lanes. Binding experiments with wild-type Rpn10 (c) and Rad23 (e). Binding with corresponding versions of the receptors lacking the Ub recognition domains, with engineered block substitution of the UIM domain (LAMAL → NNNNN) of Rpn10 (d) or deletion of the UBA domains of Rad 23 (f). Image published in: Dimova NV et al. (2012) Image downloaded from an Open Access article in PubMed Central. Image reproduced on Xenbase with permission of the publisher and the copyright holder. Larger Image Printer Friendly View |
