XB-IMG-126042
Xenbase Image ID: 126042
|
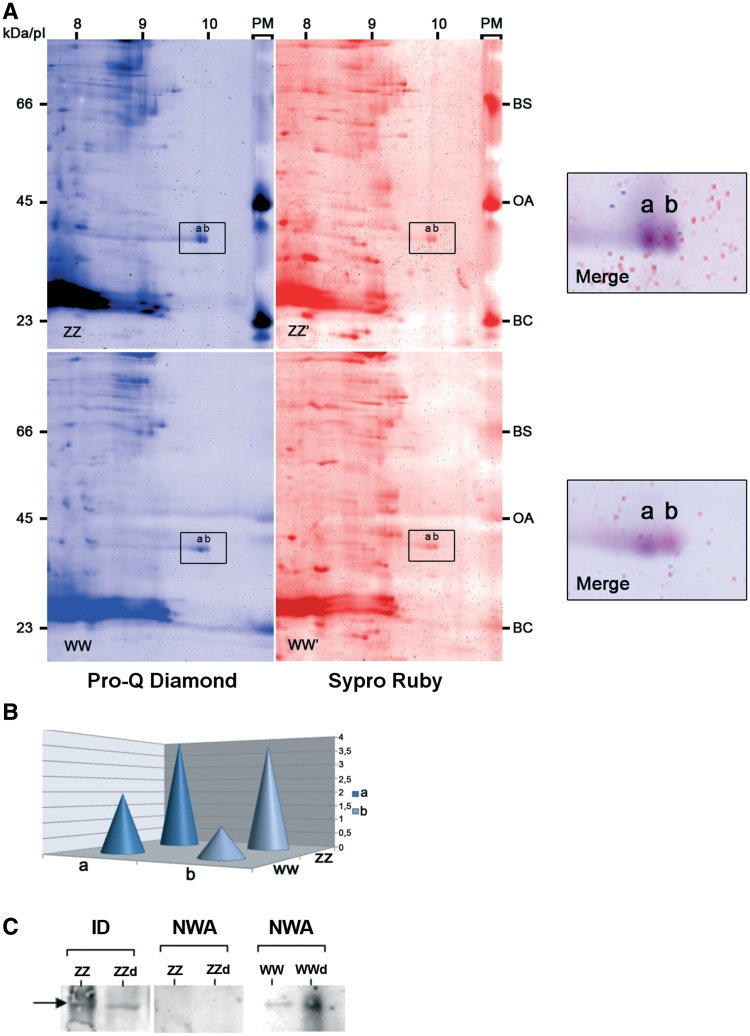
Figure 6. Relationship between PwhnRNPG phosphorylation state and RNA-binding activity. (A) Protein extracts of ZZ and WW GVs were separated in 2D-gels and stained with two sequential fluorescent dyes. Pro-Q Diamond (in blue) was specific of the phosphorylated proteins and Sypro Ruby (in red) revealed all proteins. A molecular mass indicator, PeppermintStick (PM), which contained phosphorylated proteins, β-casein (BC), ovalbumin (OA) and an non-phosphorylated protein, bovine serum albumin (BA) was run in parallel with each gel to show the specificity of the staining. The box indicates the major forms (a and b) of PwhnRNP G at pI 10. A high magnification of the merge of the two fluorescent stains is shown on the side of each gel. (B) Representative quantification of the phosphorylation degree of the major forms of PwhnRNP G (a and b) based on the intensity of their fluorescent staining by Pro-Q Diamond. Values were normalized relatively to the protein quantity estimated from the Sypro Ruby staining. (C) RNA-binding activity of dephosphorylated PwhnRNP G. Dephosphorylated ZZ (ZZd) and WW (WWd) or non-dephosphorylated (ZZ and WW) GV extracts were submitted to monodimensional NWA using WEc RNA probe followed by immunodetection (ID) of PwhnRNP G using the anti-hnRNP G serum. The arrow points to the PwhnRNPG protein. Note that a band corresponding to PwhnRNP G was immunodetected in ZZ GV, although no signal was visible in the corresponding blot after NWA in dephosphorylated or non-dephosphorylated extracts. Image published in: Kanhoush R et al. (2011) © The Author(s) 2011. Creative Commons Attribution-NonCommercial license Larger Image Printer Friendly View |
