XB-IMG-122701
Xenbase Image ID: 122701
|
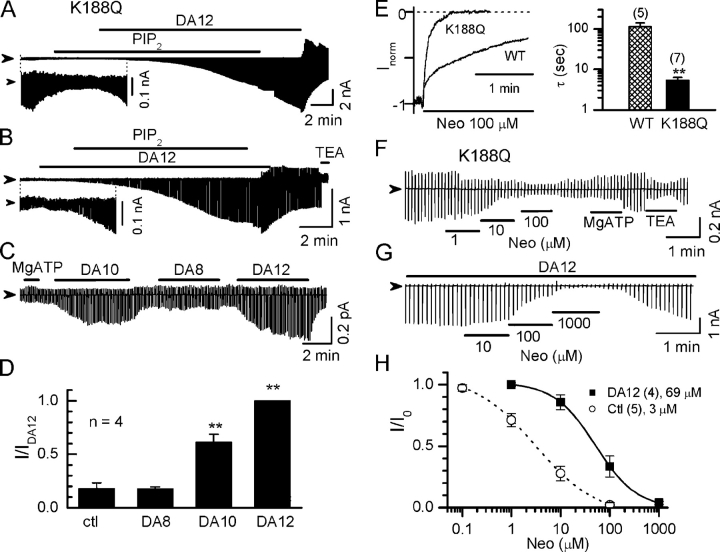
Figure 4. Long polyamines strengthen the PIP2–channel interaction in Kir2.1/K188Q channels. (A and B) Current traces recorded from the K188Q channels, which have reduced PIP2 binding affinity. Strong channel activation was observed only in the presence of both PIP2 (10 μM) and long diamine (100 μM DA12). The inset in each panel shows the current traces with expanded ordinate scale for clarity. (C) Long polyamines (DA12 and DA10) enhanced channel activity after MgATP treatment. Application of 2 mM MgATP and 100 μM diamines of various lengths are indicated by the horizontal bars above the current traces. (D) Summary of the data shown in the C. The currents are normalized to that in the presence of DA12 (IDA12). **, P < 0.05 compared with ctl. (E) Comparison of time course of neomycin (10 μM) inhibition on Kir2.1 wild type (WT) versus K188Q mutation. Currents were recorded at a holding potential of −80 mV. (F and G) Current traces recorded from K188Q channels showing sensitivities to neomycin under control conditions (ctl, in the absence of any polyamine) and in the presence of 100 μM DA12, respectively. (H) Dose–response curve of normalized current (I/I0) versus neomycin concentration in the absence and presence of 100 μM DA12 in K188Q channels. I0 represents current level at −100 mV before perfusion of neomycin. Number of patches and mean IC50 are indicated in the legends. Image published in: Xie LH et al. (2005) Copyright © 2005, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
