XB-IMG-118907
Xenbase Image ID: 118907
|
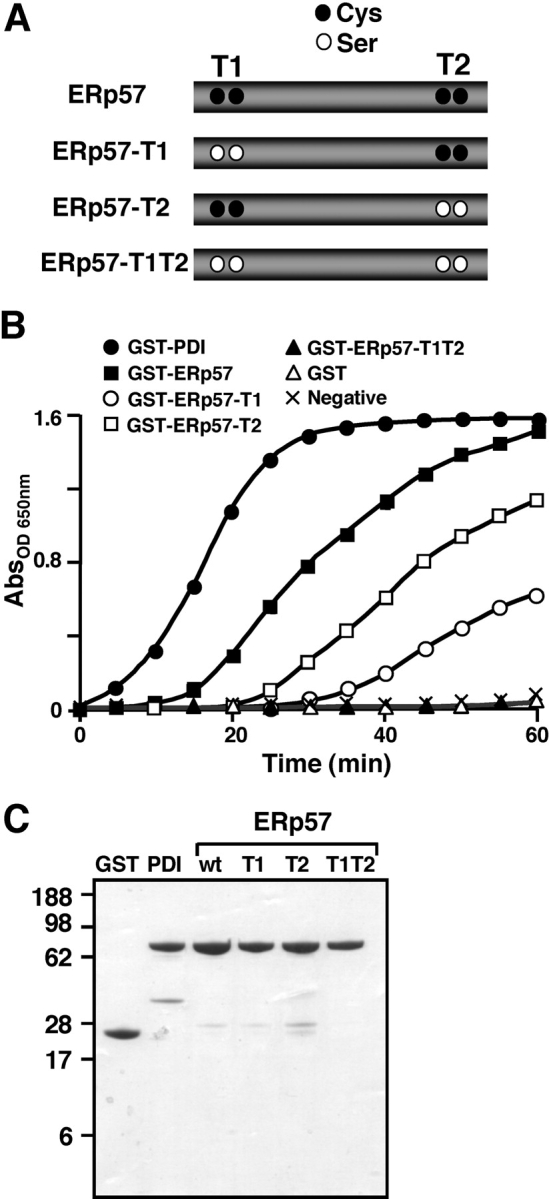
Figure 3. Enzymatic activity of ERp57 and mutants measured in vitro. (A) ERp57 mutants lacking thioredoxin motifs. Two conserved WCGHCK motifs of ERp57 are thought to be the active sites for thiol-dependent oxidoreductase activity. Cysteines in each motif are indicated in black circles. Mutagenesis of relevant cysteines into serines is indicated by white circles. (B) Thiol-dependent catalytic activity of GST-PDI (positive control) and GST-ERp57 or mutant GST-ERp57 fusion proteins measured in vitro by the insulin turbidity assay at 300 μM [Ca2+]. GST alone and lack of enzyme input are used as negative controls in this assay. Triplicate absorbanceOD 650 values were taken in three independent experiments and plotted as a function of time. Input amount of GST fusion proteins was 0.8 μM except for GST (22.04 μM). (C) Coomassie blue staining of GST and GST fusion proteins as labeled after one-step affinity purification. Proteins (5 μg each) were resolved through 12% SDS-PAGE. Image published in: Li Y and Camacho P (2004) Copyright © 2004, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
