XB-IMG-127973
Xenbase Image ID: 127973
|
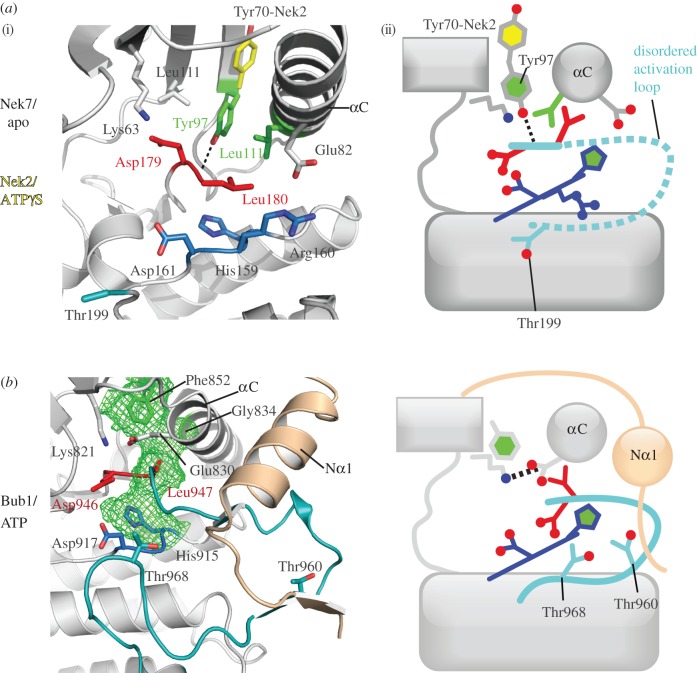
Figure 5. The β4-strand component of the hydrophobic spine is displaced in Nek7 and Bub1. (i) The structures in cartoon representation, with key residues shown as sticks; (ii) the same information in a schematic representation, based on figure 2c. (a) The structure of Nek7 (PDB code 2WQM) reveals an autoinhibited ‘Tyr-down’ conformation, in which the side chain of Tyr97, the β4-strand component of the hydrophobic spine, points into the active site, which displaces the C-helix from its active position. In order for Nek7 to be catalytically active, Tyr97 must change conformation to adopt the position shown for the equivalent side chain in Nek2 (Tyr70, yellow, PDB code 2W5B). (b) The structure of Bub1 (PDB code 3E7E) reveals a hydrophobic spine comprising only three side chains (green mesh). Phe852 (which is equivalent to Tyr97 on Nek7) adopts the down position, but this does not disrupt the Lys–Glu pair, and a continuous hydrophobic spine is formed (green mesh). This is because the side chain of Phe852 fills the void that is left by the absence of a side chain on the αC component of the spine (Gly834). The extended N-terminus of the Bub1 kinase domain (beige), which is crucial for activity, interacts with the activation segment. Thr960 and Thr968 are candidates to form interactions with Asp917 in the active conformation. Image published in: Bayliss R et al. (2012) . Creative Commons Attribution license Larger Image Printer Friendly View |
