XB-IMG-123406
Xenbase Image ID: 123406
|
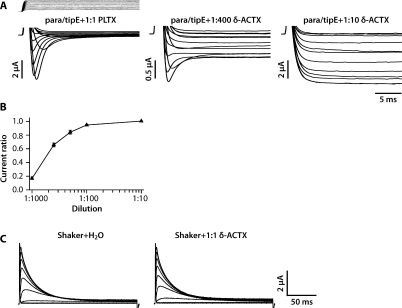
Figure 3. Membrane-Tethered δ-ACTX-Hv1a Abolishes Inactivation of Drosophila para Voltage-Gated Na+ ChannelXenopus oocytes were injected with same ratio of para/tipE cRNA by weight (1.66/1.32 ng/nl), and inward Na+ currents were recorded in response to step depolarizations from holding potential of −90 mV to a series of test potentials from −70 mV to 40 mV in 10 mV increments.(A) Representative recordings from oocytes injected with cRNA encoding para/tipE and membrane-tethered PLTXII Ca2+ channel toxin or membrane-tethered δ-ACTX-Hv1a Na+ channel toxin. Para/tipE conducts inward Na+ current exhibiting fast activation and inactivation. Membrane-tethered δ-ACTX-Hv1a totally abolishes inactivation of the current when injected at 1:10, and partially abolishes inactivation at 1:400 dilution. Large transient capacitative currents at the beginning and end of the voltage steps were cropped. Current ratio refers to the ratio of steady state current to peak current for the depolarizing step to −30 mV.(B) The curve shows the relationship between membrane-tethered toxin cRNA dilution ratio and current ratio (Iss/IP). Membrane-tethered δ-ACTX -Hv1a inhibits the inactivation of para Na+ channel in a dose-dependent manner.(C) Representative recordings from oocytes injected with cRNA encoding Drosophila Shaker K+ channel and membrane-tethered δ-ACTX-Hv1a or H2O. Shaker conducts outward K+ current exhibiting fast activation and inactivation. Membrane-tethered δ-ACTX-Hv1a has no effect on Shaker inactivation. Image published in: Wu Y et al. (2008) Copyright: © 2008 Wu et al. Creative Commons Attribution license Larger Image Printer Friendly View |
