XB-IMG-136678
Xenbase Image ID: 136678
|
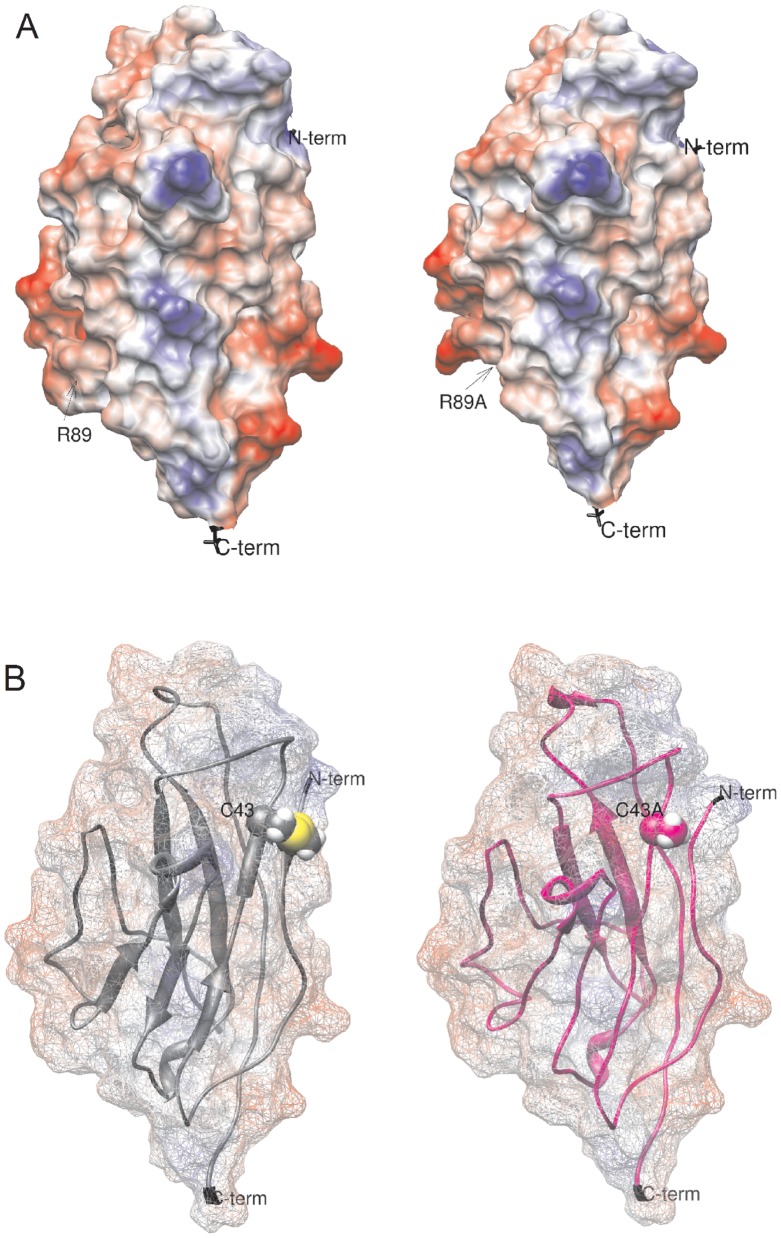
Figure 5. Effects of β1 single residue substitutions on the electrostatic surface potential (ESP).(A) ESP of the extracellular domain of WT β1 (left) and the R89A mutant (right). The point mutation increased the protein’s negative charge by the loss of a salt bridge between Arg89 and Glu87. The acidic carboxylic side chain was now exposed and reactive. (B) ESP of WT (left) and the C43A mutant (right). This substitution prevented the formation of an intermolecular disulphide bond between C43 and C21, increasing entropy. Calculations were made under the AMBER-Gasteiger force field in Chimera 1.3.5 after energy minimization under AMMP in VEGA ZZ 2.3.2. Image published in: Islas AA et al. (2013) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution license Larger Image Printer Friendly View |
