XB-IMG-155574
Xenbase Image ID: 155574
|
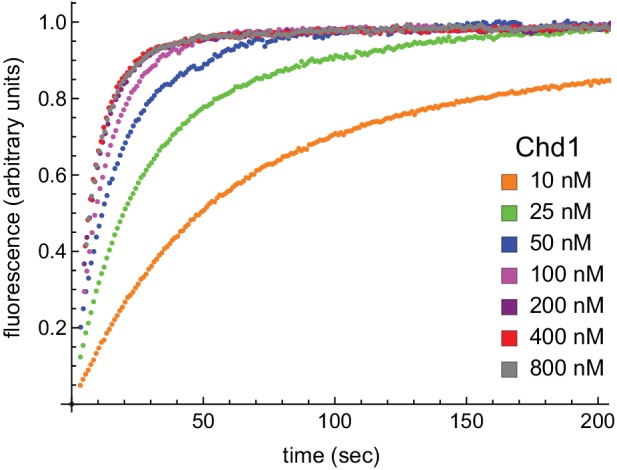
Figure 7—figure supplement 2. With limiting ATP, remodeling saturates at 400 nM Chd1.Nucleosomes formed by adding 12 nM dimer to 10 nM 0-601-80 hexasomes were titrated with 10, 25, 50, 100, 200, 400, and 800 nM Chd1 and 25 μM ATP. Reactions were monitored by Cy3-Cy3 SQOF via fluorometer, and show that remodeling plateaued at 400 nM Chd1. The progress curves shown are representative of two independent Chd1 titrations using unmodified H2B (Wt-Wt). Similar results were observed for duplicate titrations using Ub-Wt, Wt-Ub, and Ub-Ub nucleosomes.DOI:
http://dx.doi.org/10.7554/eLife.21356.015 Image published in: Levendosky RF et al. (2016) © 2016, Levendosky et al. Creative Commons Attribution license Larger Image Printer Friendly View |
