XB-IMG-117807
Xenbase Image ID: 117807
|
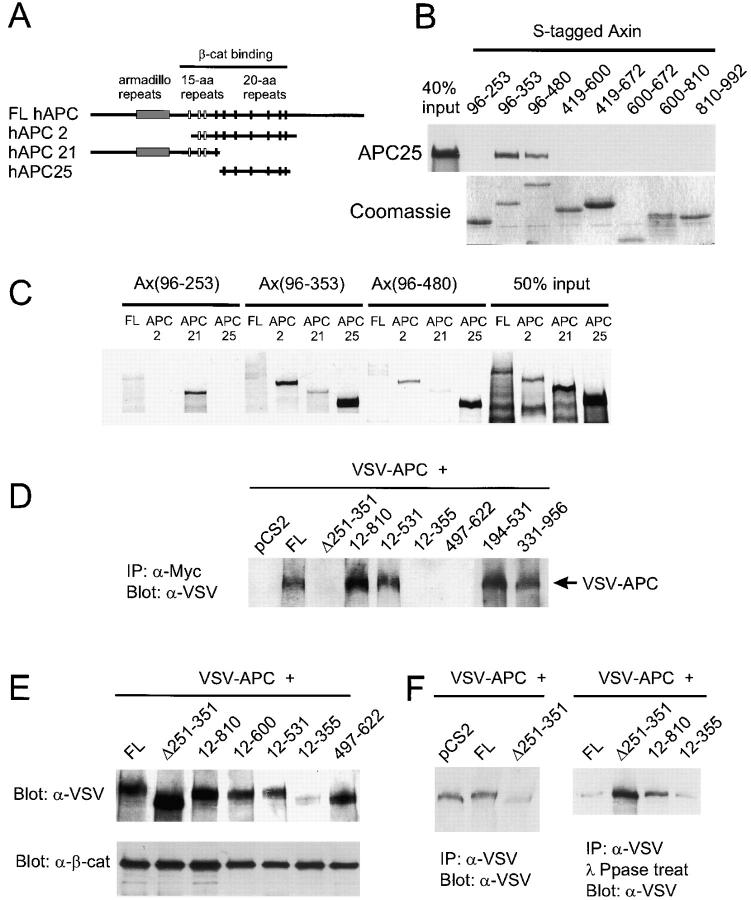
Figure 2. Axin binds to APC and induces APC phosphorylation in vivo. (A) Diagram showing the regions of human APC included in three APC constructs. (B) Direct in vitro binding of APC25 to Axin fragments containing the RGS domain. Sulfur-35–labeled APC25 protein was incubated with S-tagged fusion proteins containing the indicated regions of Axin. After S-protein agarose IP, bound APC25 was detected by SDS-PAGE and autoradiography. Coomassie blue staining of the Axin fusion proteins is shown at the bottom. (C) In vitro binding with NH2-terminal Axin fragments reveals a second APC-binding site upstream of the RGS domain. Binding of APC2 and APC25 required the RGS domain (Ax96-353 and Ax96-480). However, FL hAPC also interacted with Ax96-253, and hAPC2 bound Ax96-253 very strongly. Interaction of Sulfur-35–labeled FL APC, APC2, APC21 and APC25 with S-tagged fusion proteins was determined as in B. (D) CoIP of VSV–APC with FL Axin and several mutant forms of Axin. 293 cells were cotransfected with VSV-G–tagged Xenopus APC (VSV–APC) and the indicated Axin constructs. The cell lysates were immunoprecipitated with anti-Myc and probed with anti–VSV-G antibody. Failure to detect interaction with Ax12-355 was due to the very low levels of VSV–APC observed when coexpressed with this particular construct (E). (E and F) Axin-dependent phosphorylation of APC. (E) Axin-induced mobility shift of VSV–APC. Total lysates from cotransfected cells were analyzed by Western blot using an anti-VSV mAb. FL Axin and mutants Ax12-810, 12-600, and 12-531 induced a mobility shift, whereas AxΔ251-351, Ax12-355, and Ax497-622 did not. (F) Phosphatase treatment eliminated the mobility shift. Cotransfected cell lysates were IP with anti-VSV mAb, and the products were analyzed by SDS-PAGE and Western blot before (left) or after (right) incubation with λ-protein phosphatase. Image published in: Fagotto F et al. (1999) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
