XB-IMG-124189
Xenbase Image ID: 124189
|
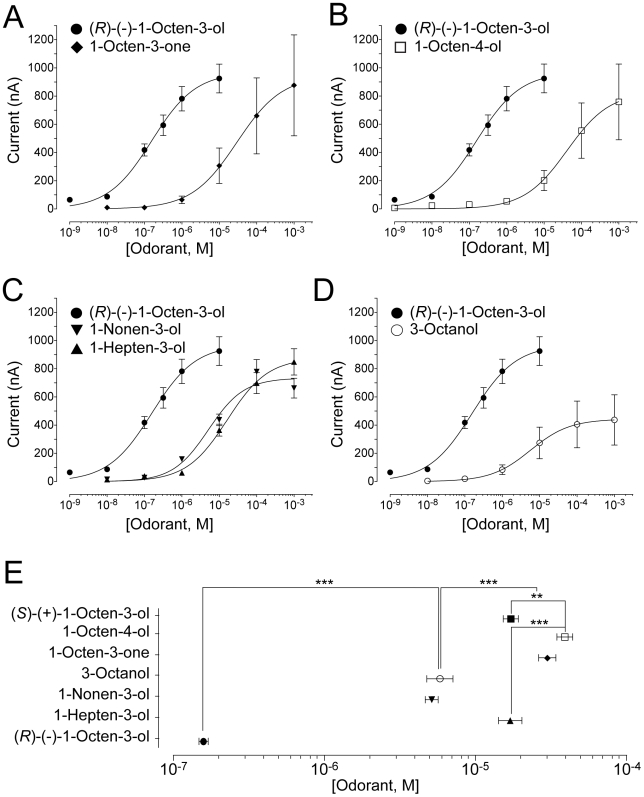
Figure 2. Strong preference of AaOR8 towards (R)-(—)-1-octen-3-ol.The concentration-response plot for (R)-(—)-1-octen-3-ol was repeated in each panel for comparative purposes. (A) Importance of C3 as a chiral center. Concentration-response plots of AaOR8 to 1-octen-3-one (n = 6). (B) Shifting the chiral center from C3 to C4 reduces AaOR8 sensitivity. Concentration-response plots of AaOR8 to 1-octen-4-ol (n = 8). (C) Side chain length affects AaOR8 sensitivity. Concentration-response plots of AaOR8 to 1-nonen-3-ol and 1-hepten-3-ol (n = 8 to 9). (D) The double bond is critical for recognition by AaOR8. Concentration-response plots of AaOR8 to 3-octanol (n = 6). (E) EC50 ranking profile of AaOR8 for octenol related compounds. Asterisk, p<0.05; two asterisks, p<0.01 and three asterisks, p<0.001. Odorant concentrations were plotted on a logarithmic scale. Each point represents the mean and error bars indicate s.e.m. Image published in: Bohbot JD and Dickens JC (2009) Image downloaded from an Open Access article in PubMed Central. This is an open-access article distributed under the terms of the Creative Commons Public Domain declaration which stipulates that, once placed in the public domain, this work may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. Larger Image Printer Friendly View |
