XB-IMG-121116
Xenbase Image ID: 121116
|
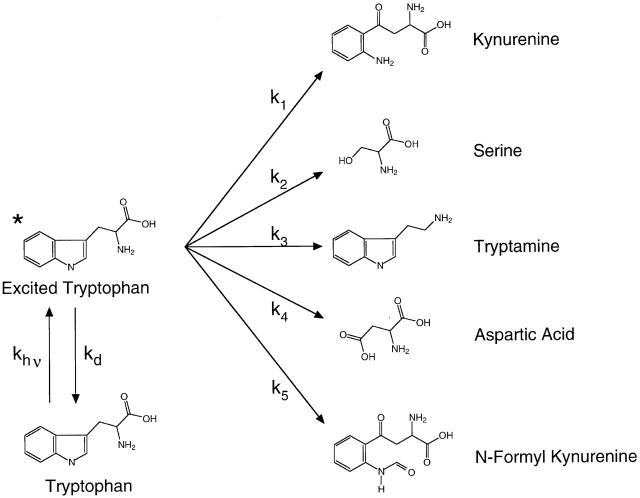
Figure 1. Schematic for photochemical modification of tryptophan. At room temperature, tryptophan molecules occupy the electronic ground state almost exclusively (bottom left). Absorption of a UV photon promotes tryptophan to an excited electronic state (*). The lifetime of the excited state is several nanoseconds (Beechem and Brand 1985). The excited tryptophan may decay back to the ground state by a number of paths; the sum of the rate constants for these deactivation pathways is denoted kd. Alternatively, the reactive excited molecule may form a covalently modified product by deactivating along one of the photochemical reaction pathways indicated by the rate constants k1 through k5. The major products of the UV photolysis of free tryptophan in aqueous solution include kynurenine, serine, tryptamine, aspartic acid, and N-formyl kynurenine (Creed 1984a). The photochemical reactivity depicted here is a general property of organic molecules. We exploited this reactivity by using UV light to “mutagenize” functional ion channel proteins in situ while observing simultaneously the effect on macroscopic ionic currents through the channels. Image published in: Middendorf TR et al. (2000) © 2000 The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
