XB-IMG-126265
Xenbase Image ID: 126265
|
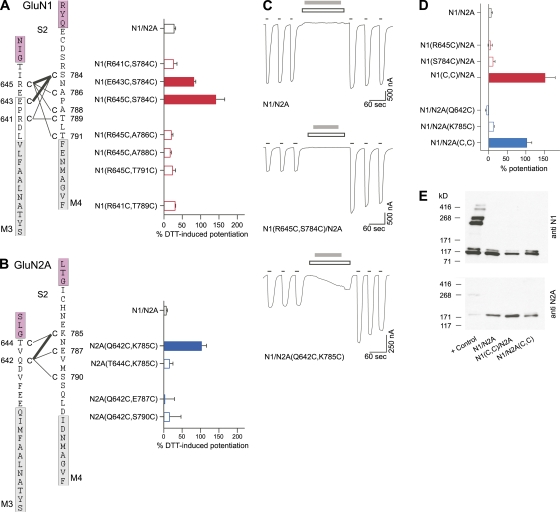
Figure 2. DTT-induced potentiation of macroscopic currents in NMDA receptors containing intrasubunit double-cysteine substitutions in GluN1 or GluN2A. (A and B) NMDA receptors with intrasubunit GluN1- or GluN2A-specific double-cysteine substitutions were assayed for DTT-induced changes in macroscopic current amplitudes using two-microelectrode voltage clamp in Xenopus oocytes. Double-cysteine–substituted GluN1 (A) or GluN2A (B) subunits were coexpressed with WT GluN2A or GluN1 subunits, respectively. (Left) Schematic representation of regions around M3–S2 and S2–M4 linkers. Positions substituted with cysteine are indicated with a “C” and numbered next to the endogenous residue. Tested pairs of cysteines are shown with a connecting line. Darker lines indicate pairs that showed significant DTT-induced current potentiation relative to GluN1/GluN2A and hence can presumably spontaneously cross-link. Numbering is for the mature protein. Proximal parts of S2 and the hydrophobic segments M3 and M4 are colored as magenta and gray, respectively. Boxed regions around the hydrophobic segments represent the α-helical extent of the transmembrane segments in an AMPA receptor structure (Sobolevsky et al., 2009). (Right) Mean percent change (±SEM; n ≥ 4) of current amplitudes after DTT. In the recording protocol for the GluN1 double-cysteine substitutions, (A) DTT was applied continuously in the presence and absence of agonists for at least 2 min (raw recordings not depicted). The recording protocol for the GluN2A double-cysteine substitutions (B) was identical to those in C. Filled bars indicate values significantly different from those of WT receptors (P < 0.05). Our experiments focused on GluN1(R645C,S784C)/GluN2A and GluN1/GluN2A(Q642C,K785C) receptors. (C) Representative membrane currents (holding potential, −60 mV) in Xenopus oocytes injected with WT GluN1/GluN2A, GluN1(R645C,S784C)/GluN2A, or GluN1/GluN2A(Q642C,K785C) receptors. Hereafter, GluN1(R645C,S784C) and GluN2A(Q642C,K785C) are referred to as GluN1(C,C) and GluN2A(C,C), respectively. Currents were elicited by coapplication of 20 µM glycine and 200 µM glutamate (thin black lines). 4 mM DTT (2 min; gray bars), applied in the presence of competitive antagonists DCKA (10 µM) and APV (100 µM) (open boxes), strongly potentiated subsequent current amplitudes in the double-cysteine–substituted receptors. (D) Mean percent change (±SEM; n ≥ 4) of current amplitudes after DTT. Filled bars indicate values significantly different from those of WT receptors (P < 0.05). (E) Western blot analysis of membrane proteins purified from Xenopus oocytes under nonreducing conditions. Formation of intersubunit cross-linking, either homomeric or heteromeric, was assayed with anti-GluN1 (top) or anti-GluN2A (bottom) antibodies. The “+ Control” is GluN1(N521C,L777C)/GluN2A(E516C,L780C) receptors that form intersubunit dimers (Furukawa et al., 2005). Expected molecular weights are: monomeric GluN1 (114 kD) and GluN2A (173 kD), homodimeric GluN1 (228 kD) and GluN2A (346 kD), and heterodimeric GluN1/GluN2A (287 kD). Other than the monomeric bands, no apparent homomeric or heteromeric dimer bands were detected for GluN1/GluN2A or either of the double-cysteine–substituted receptors, although dimers were present for the “+ Control” (n = 4). Image published in: Talukder I and Wollmuth LP (2011) © 2011 Talukder and Wollmuth. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
