XB-IMG-123683
Xenbase Image ID: 123683
|
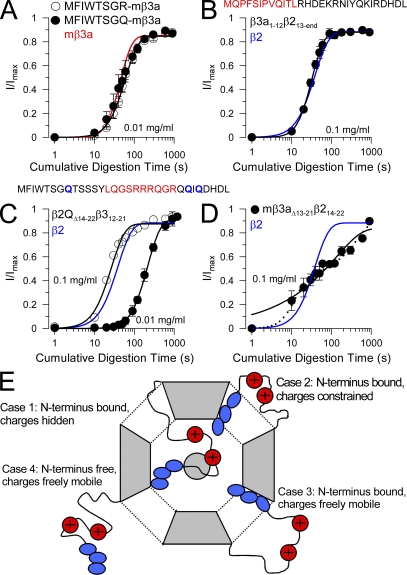
Figure 9. Exchange of hydrophobic and charged segments between β2 and hβ3a N termini. (A) The time courses of digestion under closed-channel conditions (0 Ca2+; 0 mV) of the MFIWTSGR-β3a and MFIWTSGQ-β3a constructs are compared with wild-type mβ3a (red line) for 0.01 mg/ml trypsin. (B) The time course of digestion of β3a1-12β213-end (MQPFSIPVQITL from β3a appended to β2 at position 13) with 0.1 mg/ml trypsin is compared with the β2 digestion time course. (C) A β3a segment (residues 12–21 containing R16-18 and R21) replaced residues 14–22 in a β2 construct in which all N-terminal basic residues were replaced with Q. The plot shows the digestion time course for both 0.1 and 0.01 mg/ml trypsin compared with that of β2 (blue line). (D) The time course of digestion with 0.1 mg/ml trypsin of construct mβ3a13-21β214-22 is compared with that of β2 (blue line). Dotted line represents a two-component exponential fit (analogous to Eq. 1) to the digestion time course, and the solid represents a fit of Eq. 1. (E) Possible configurations of N termini in a closed channel are schematized. Case 1, an N terminus is bound in the antechamber, and basic residues are shielded from digestion; Case 2, the N terminus is bound in the antechamber, but, although basic residues are outside the antechamber, they are structurally constrained, thereby hindering digestion; Case 3, the N terminus is bound in the antechamber, but basic residues are freely mobile and accessible to attack by trypsin; Case 4, an N terminus is freely mobile outside the antechamber allowing easy digestion by trypsin. Image published in: Zhang Z et al. (2009) © 2009 Zhang et al. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
