XB-IMG-120210
Xenbase Image ID: 120210
|
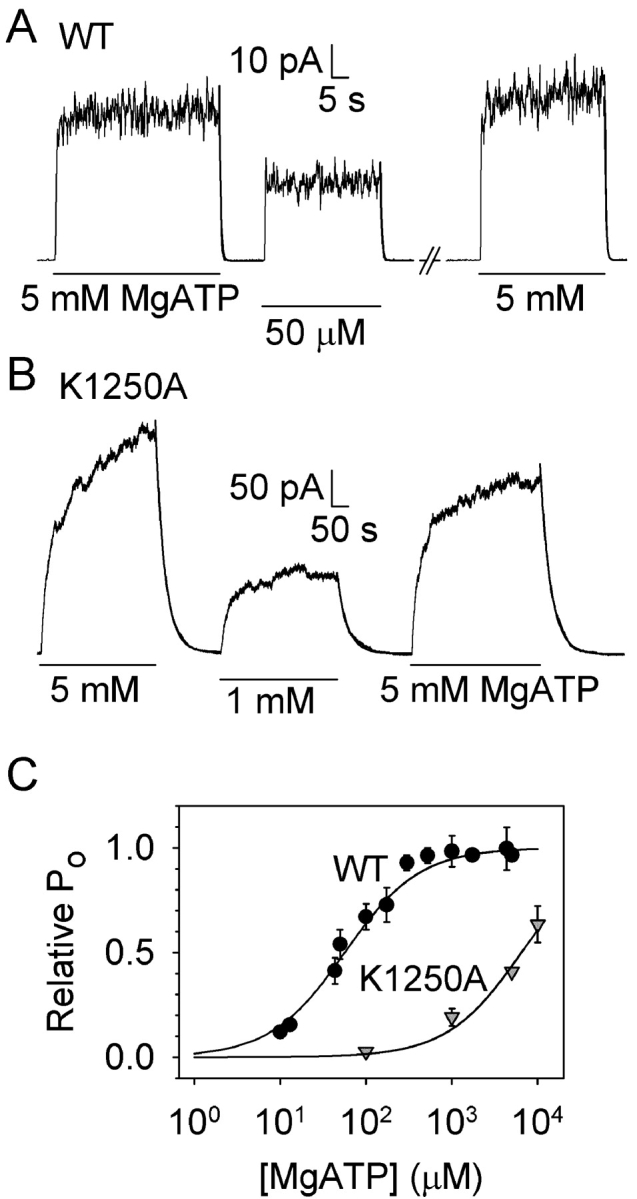
Figure 3. . The K1250A mutation strongly shifts the [MgATP] dependence of Po to higher [MgATP]. (A) steady state level of macroscopic current of prephosphorylated WT CFTR channels was ∼2-fold lower at 50 μM MgATP than during bracketing exposures to 5 mM MgATP (as expected from Fig. 2); lines below traces mark MgATP applications. Rapid current decay on MgATP washout gave (exponential fit lines superimposed on traces) τ = 0.45 s, τ = 0.40 s, τ = 0.38 s, from left to right (mean τ = 0.54 ± 0.04 s, n = 21, pooled from all [MgATP]). (B) Macroscopic current of K1250A channels was reduced ≥2-fold on lowering [MgATP] from 5 to 1 mM. Superimposed exponential fit lines show slower current decay (note 10-fold contracted time scale relative to A) with, from left to right, τ = 28 s, τ = 30 s, τ = 32 s (mean τ = 39 ± 5, n = 9, from all [MgATP]). (C) Semilog plot of Po versus [MgATP]. Steady currents (averaged over final ≥20 s) at each [MgATP], normalized to the mean bracketing level at 5 mM MgATP, yielded least-squares. Michaelis fit parameters for WT: Po max = 1.04 ± 0.01, K0.5 = 57 ± 2 μM; for K1250A: Po max = 2.45 ± 0.88, K0.5 = 6.5 ± 4.8 mM; for display, WT (circles) and K1250A (inverted triangles) data (mean ± SD, 3 ≤ n ≤9) were renormalized to these Po max values. Because 10 mM, the highest [MgATP] used, was still far from saturating for K1250A channels, the fit for this mutant is less accurate, evident from large errors on fit parameters. Image published in: Vergani P et al. (2003) Copyright © 2003, The Rockefeller University Press. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
