XB-IMG-117773
Xenbase Image ID: 117773
|
|
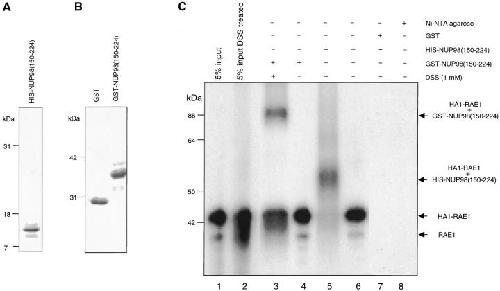
Figure 2. Chemical cross-linking of in vitro–translated HA1-RAE1 to E. coli–purified GLEBS-like motifs. (A) Purified recombinant HIS-NUP98(150–224) (∼11-kD) protein separated by SDS-PAGE (15% polyacrylamide gel) and detected by Coomassie brilliant blue (CBB; Bio-Rad Laboratories) staining. (B) Purified recombinant GST (29 kD) and GST-NUP98(150–224) (∼38 kD) protein run on a 10% polyacrylamide gel and stained with CBB. (C) Pull-down assays performed with [35S]-methionine–labeled HA1-RAE1 synthesized in vitro (45 kD), and GST- or HIS-NUP98(150–224) fusion proteins purified from E. coli. 5% input shows 5% of the labeled HA1-RAE1 protein used in each pull-down assay. Typically, the in vitro–translated RAE1 appears as a doublet, representing fragments with and without a HA1 tag (the RAE1 cDNA was cloned in pSP73 as a HA1 fusion gene that retained the endogenous RAE1 translation initiation codon). GST beads and Ni-NTA agarose acted as negative control for binding in GST-NUP98(150–224) and HIS-NUP98(150–224) pull-down assays, respectively. Comparable amounts of GST, GST-NUP98(150–224), and HIS-NUP98(150–224) proteins were used in each pull-down assay. The experiment shown is representative for two independent experiments. A cross-linked GST-NUP98(150–224)/RAE1 product of ∼84 kD and a cross-linked HIS-NUP98(150–224)/RAE1 product of ∼56 kD were obtained specifically in DSS-treated samples. Note that cross-linking of RAE1 to HIS-NUP98(150–224) was more efficient than to GST-NUP98(150–224). Molecular weight standards are indicated at right. Image published in: Pritchard CE et al. (1999) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
