XB-IMG-194787
Xenbase Image ID: 194787
|
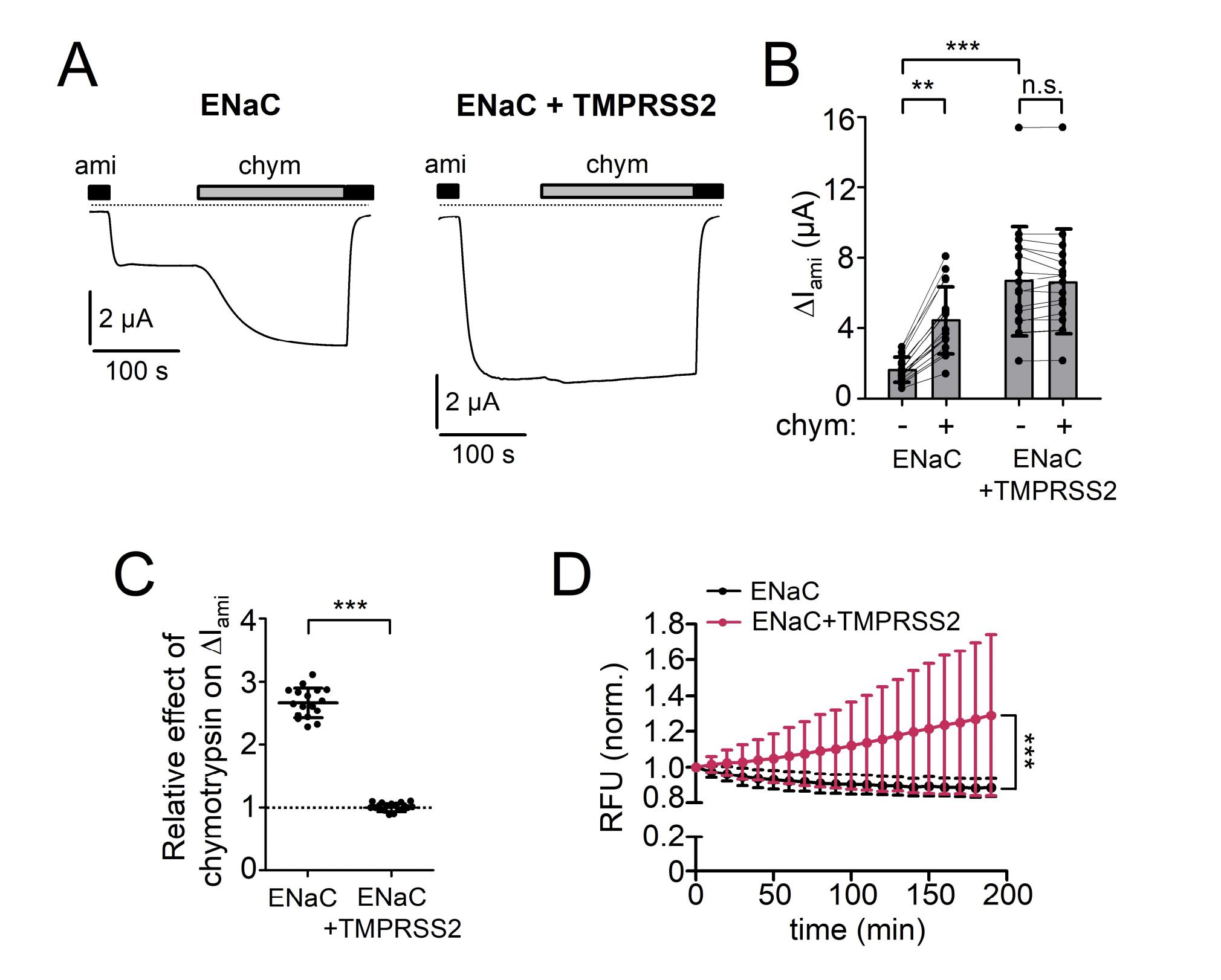
Figure S1. TMPRSS2 without C-terminal HA-tag also proteolytically activates αβγ-ENaC. (A)
representative whole-cell current traces recorded in a human αβγ-ENaC expressing oocyte without (left
trace, ENaC) or with (right trace, ENaC+TMPRSS2) human TMPRSS2 coexpression. In these control
experiments a TMPRSS2 construct was used without HA-tag attached to its C-terminus. Amiloride (ami,
2 µM) and chymotrypsin (chym, 2 µg/ml) were present in the bath solution as indicated by black and
grey bars, respectively. Dashed lines indicate zero current level. (B) ENaC-mediated amiloride-sensitive
whole-cell currents (ΔIami) were determined as described in Fig. 1B from similar experiments as shown
in (A). Lines connect data points obtained in an individual oocyte. Mean ± SD and data points for
individual oocytes are shown; ***p < 0.001; **p < 0.01; n.s., not significant, Kruskall-Wallis with
Dunn’s post hoc test (n=17, N=3). (C) relative stimulatory effect of chymotrypsin on ΔIami summarized
from data shown in (B). Dashed line indicates a normalized ΔIami value of one (no effect). Mean ± SD
and data points for individual oocytes are shown; ***p < 0.001; two-tailed unpaired Student’s t test. (D)
In parallel experiments to those shown in (A-C), trypsin-like proteolytic activity at the cell surface (RFU
= relative fluorescent unit; mean ± SD) was detected in these batches of oocytes as described in Fig. 1D.
***p < 0.001; two-tailed Mann-Whitney test (at the time point 190 min; n=22, N=3). Image published in: Sure F et al. (2022) © 2022 The Authors. Creative Commons Attribution license Larger Image Printer Friendly View |
