XB-IMG-117889
Xenbase Image ID: 117889
|
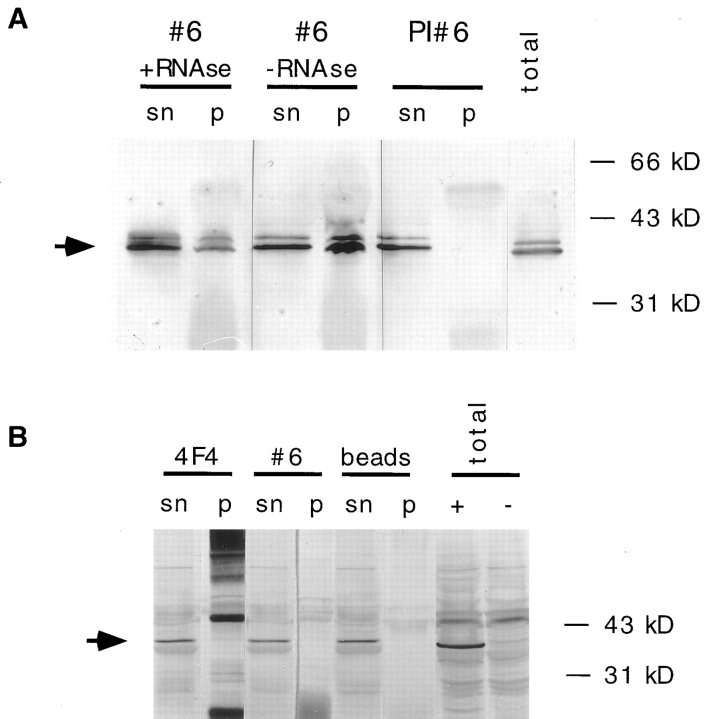
Figure 10. Association of Xlrbpa with hnRNPs. (A) HeLa nuclear
extracts were used for immunoprecipitations with anti-Xlrbpa
serum Rb6 (#6), or the corresponding preimmuneserum (PI #6).
Some of the material was digested with RNases (+RNAse) or
left undigested (−RNAse) before incubation with the antibody-coupled beads. Before washing, the beads were pelleted by centrifugation and an aliquot of the supernatant was saved (sn). After several washes, beads were boiled in SDS sample buffer, and
corresponding supernatants (sn) and pellets (p) were assayed for
the presence of hnRNPs C1 and C2 by Western blotting with
mAb 4F4. As a reference, total HeLa nucleoplasmic extracts
were loaded (total). Antiserum Rb6 (#6) could coprecipitate
hnRNPs C1 and C2 while the corresponding preimmuneserum did
not. Coprecipitation of hnRNPs was resistant to RNase digestion
(+RNAse), indicating a physical association of Xlrbpa with these
proteins. An arrow indicates the position of hnRNPs C1 and C2.
(B) Immunoprecipitations of nuclear extracts from HeLa cells
stably expressing MP2 protein performed with anti-hnRNP antibody 4F4 (4F4), antiserum Rb6 (#6) or beads alone (beads). The
precipitated material (p) and corresponding supernatants (sn)
were tested for the presence of MP2 protein by Western blotting
with mAb 9E10. No MP2 protein could be coprecipitated with either antibody, instead all MP2 protein remained in the supernatant. As a control total extracts of HeLa cells (total) expressing
MP2 (+) or untransfected cells (−) were loaded. An arrow indicates position of MP2 protein. The strong background bands in
the 4F4 pellet lanes derive from IgG heavy and light chains,
which are detected by the secondary anti–mouse antibody used to
detect mAb 9E10. Image published in: Eckmann CR and Jantsch MF (1997) Image reproduced on Xenbase with permission of the publisher and the copyright holder. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
