XB-IMG-116895
Xenbase Image ID: 116895
|
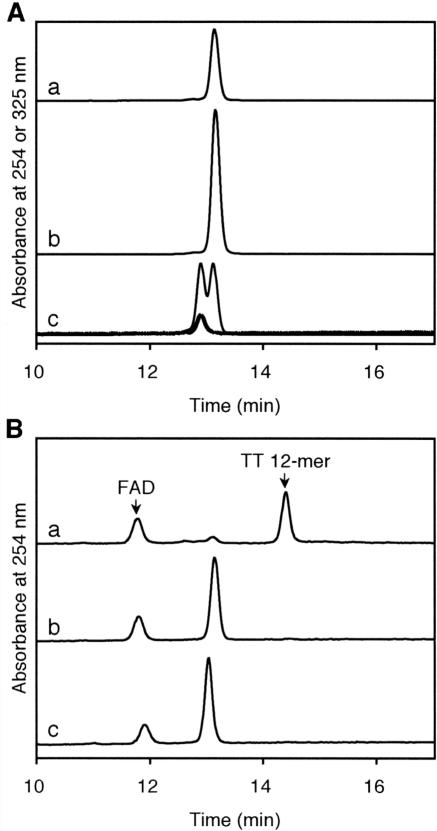
Figure 4. (A) Reversed-phase HPLC analysis of the Dewar 12mer. (a) The 12mer synthesized in this study, (b) co-injection with the Dewar photoproduct-containing 12mer prepared by irradiation of the (6–4) photoproduct-containing 12mer and (c) co-injection with the (6–4) photoproduct-containing 12mer with the same sequence context. The thick and thin lines show the chromatograms monitored at 325 and 254 nm, respectively, and the 325 nm chromatogram is magnified by a factor of 5. (B) Analysis of the (6–4) photolyase reaction with the (6–4) 12mer (a) and the Dewar 12mer (b and c). The reaction times were 3 h (a and b) and 24 h (c). The HPLC conditions are described in Materials and Methods. Image published in: Yamamoto J et al. (2006) © 2006 The Author(s). Creative Commons Attribution-NonCommercial license Larger Image Printer Friendly View |
