XB-IMG-122910
Xenbase Image ID: 122910
|
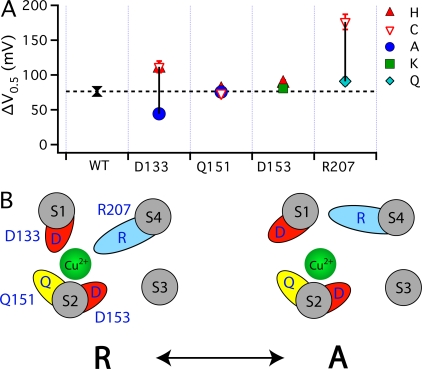
Figure 9. Effect of mutations on Cu2+-efficacy. (A) The maximal GK-V shift (ΔV0.5MAX) in response to saturating 1000 μM Cu2+ are plotted for the WT () and mutants at the four putative Cu2+-coordinating sites (Ala: •; Lys: ▪; Gln: ⋄; Cys: ▿; His: ▴). The dashed line indicates ΔV0.5MAX for the WT. The vertical solid lines indicate the range of ΔV0.5MAX for mutants at each position. (B) A speculative model of the Cu2+ binding site in the resting (R) and activated (A) state of the voltage sensor. In the resting state, Cu2+ is coordinated by D133(S1), Q151(S2), D153(S2), and R207(S4). The relative size and position of transmembrane segments correspond to those in the structure of a Kv1.2/Kv2.1 chimera (Long et al., 2007). During activation, the S2 residues interact with Cu2+ in a state-independent manner while interactions with D133 and R207 are weakened or disrupted, presumably by changes in the position or orientation of S1 and S4 relative to S2. In this way, mutation of any of these residues alter IC50, but only mutation of D133 or R207 alter efficacy. Image published in: Ma Z et al. (2008) © 2008 Ma et al. Creative Commons Attribution-NonCommercial-ShareAlike license Larger Image Printer Friendly View |
